
More at Medical News

"These findings fill a gap that existed previously in terms of data on whether maintenance therapy with lenalidomide prolongs the time to disease progression after initial therapy. We now have evidence that it does, in this and the two other lenalidomide studies that are presented in this issue of the Journal," said Dr. McCarthy. "This shows that patients with multiple myeloma now have options for prolonging the response to initial therapy. The next steps will be trying to improve on these responses by adding new agents that may prove even more effective in combination with lenalidomide following transplant."
 lenalidomide
lenalidomide "Lenalidomide maintenance following autologous stem cell transplant can now be considered a standard of care for people with multiple myeloma," says Dr. McCarthy, senior author on the meta-analysis and Principal Investigator of the U.S. study, CALGB (Alliance) 100104. "The improvements over the last decade in terms of both survival and quality of life for patients with this disease are striking, and very encouraging."
"Our results are clear. Using bortezomib in combination with lenalidomide and dexamethasone in front-line treatment - hitting the disease early and hard - makes a meaningful difference for myeloma patients," said study principal investigator Dr. Durie, a physician at Cedars-Sinai Outpatient Cancer Center in Los Angeles. "Our results represent a potential new standard of care."
 bortezomib
bortezomib  lenalidomide
lenalidomide dexamethasone
dexamethasone
“Nearly 15 years following the initial FDA approval, Revlimid continues to demonstrate benefits for new patient populations,” said Jay Backstrom, M.D., M.P.H., Chief Medical Officer for Celgene. “Revlimid in combination with rituximab (R2) leads to immune-mediated treatment effects and represents a chemotherapy-free treatment option that can help patients with previously treated follicular lymphoma and marginal zone lymphoma delay disease progression.”
“Chemotherapy continues to be a standard of care for indolent forms of NHL, but most patients will relapse or become refractory to their current treatment,” said Meghan Gutierrez, Chief Executive Officer for the Lymphoma Research Foundation. “This approval represents a new therapeutic option for previously treated patients with follicular and marginal zone lymphomas, including those who relapse or no longer respond to initial treatment. We commend the patients and scientists who participated in the clinical study for advancing lymphoma research and treatment.”

The research, published in a letter to the journal Signal Transduction and Targeted Therapy, shows the potential of an existing FDA-approved drug to help doctors treat the sickest COVID-19 patients.
The labs of Pengda Liu, Ph.D., and Guochun Jiang, Ph.D., both assistant professors in the UNC Department of Biochemistry and Biophysics, conducted this work. Liu is a member of the UNC Lineberger Comprehensive Cancer Center, and Jiang is a member of the UNC HIV Cure Center.
SARS-CoV-2, a novel coronavirus and the causative agent of COVID-19, has caused a global social and economic disruption, and there are still thousands of cases and death each day in the United States and around the world. Treatments to prevent severe illness and death are still needed.
In this research letter, the UNC team reported that a protein called E3 ligase SPOP recognizes and protects the human cell surface receptor ACE2, which is the protein SARS-CoV-2 latches onto in order to gain entry into cells to cause infection. Another protein called CK1 kinase triggers this recognition and protection of ACE2.
The researchers used the cancer drug lenalidomide to inhibit CK1 kinase activity in cell cultures and showed a substantial reduction in ACE2 protein levels in kidney cancer cells. Researchers used SARS-CoV-2 S protein conditioned pseudoviruses in vitro and found lenalidomide treatment reduced the effect of this infection on kidney-derived cells.
"We hope that our identification and tests for the efficacy of inactivating the SPOP/CKI signaling in reducing ACE2 protein expression to attenuate SARS-CoV-2 infection provides a timely investigation into new therapeutic directions to combat COVID-19," Liu said.
A next step could be to use animal models to see if the drug blocks real SARS-CoV-2.
"This combination appears to deliver everything we expected and more," said Jakubowiak, who came to the University of Chicago this fall from the University of Michigan. "We have seen excellent efficacy — the best reported to date — without the neurotoxicity that has been problematic with other drug combinations."
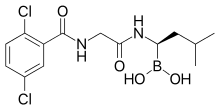

"These rapid and durable response rates are higher than those achieved by the best established regimens for newly diagnosed multiple myeloma," said Jakubowiak. "We have observed excellent efficacy, the best reported to date, and very good tolerability, including limited peripheral neuropathy that has been problematic with other drug combinations."
Fifteen of the 35 newly diagnosed patients in the open-label phase 2 portion of the study subsequently underwent autologous (using their own blood-forming stem cells) transplants, a standard treatment for multiple myeloma and did very well.
For the entire group, after a median 19.3 months of follow up, the median time-to-progression (TTP) of the disease, progression-free survival (PFS), and overall survival (OS) had not yet been reached, according to the presentation. The estimated TTP and PFS at one year are 76 percent, and the estimated one-year overall survival is 100 percent, the results showed.