Friday, July 27, 2012
Ancient berry could protect against diabetic retinopathy
Saturday, March 10, 2012
Berry fruits help the brain stay healthy in several ways
Wednesday, July 19, 2017
Animal studies examine role of raspberry products in weight management and motor function
Friday, April 16, 2010
Chinese Wolfberries (as dietary supplement) may improve vision imperfections caused by Type-2 diabetes..
It is also known as Chinese wolfberry, mede berry, barbary matrimony vine, bocksdorn, Duke of Argyll's tea tree, Murali (in India), red medlar, or matrimony vine. Unrelated to the plant's geographic origin, the names Tibetan goji and Himalayan goji are in common use in the health food market for products from this plant.
"I would not say that wolfberries are a medicine, but they can be used as a dietary supplement to traditional treatments to improve vision," Lin said. "Wolfberries have high antioxidant activity and are very beneficial to protect against oxidative stress caused by environmental stimuli and genetic mutations."
Ref : http://www.k-state.edu/media/newsreleases/mar10/wolfberry33010.html
Thursday, August 12, 2010
ProstaCaid (33-ingredient comprehensive polyherbal preparation) against prostate cancer......
Herbal extracts include the extracts from turmeric root, saw palmetto berry, grape skin, pomegranate, pumpkin seed, pygeum bark, sarsaparilla root, green tea, and Japanese knotweed. Hence, it is rich in natural polyphenols, including quercetin, resveratrol, epigallocatechin gallate (EGCG), and ellagic acid, which have previously demonstrated anticancer potential. The unique formula contains 3 medicinal mushrooms grown on an herbal-enhanced medium. The mushrooms included are Phellinus linteus, Ganoderma lucidum, and Coriolus versicolor, each with known anticancer properties.
Researchers claim that, ProstaCaid was designed based on constituents that exhibit antiprolifetaive, antioxidant, and apoptotic activities; however, its efficacy and the mechanisms of action are yet to be examined. Researchers looked at the effectiveness of the preparation in suppressing several types of prostate cancer cell lines in culture and attempt to delineate the mechanism of action for justification in pursuing animal to determine efficicacy invivo.
Researchers conclude that, the anticancer activity of ProstaCaid may be ascribed to its polyphenolic flavonoids and curcuminoids derived from various herbs as well as other supplements, such as DIM. The preparation contains supplements such as quercetin (15%), Curcuma longa root extract complex with enhanced bioavailability (BCM-95; 20%), DIM (3%), and resveratrol (0.2%). Some of these components have shown a strong doseand time-dependent growth inhibition and apoptotic death in prostate cancer cells; 25 mM of quercetin inhibited about 50% PC3 cell growth for 72 hours. At 24 hours, 50 mM and 100 mM quercetin induced G2/M arrest and apoptosis, manifested by the decrease in G2/M-related protiens.
Researchers summarise that, ProstaCaid has anti-cancer activities in both AD and AI prostate cancer cells at very low concentrations (25 mg/mL). It also suggests that ProstaCaid inhibits cell growth and survival, at least through the inhibition of AKT and MAPK signaling. The effect on AI cell lines is especially of importance as there is presently no curative therapy for hormone refractory prostate cancer.
Researchers postulate that ProstaCaid may affect activity of Cdc2/cyclin B1 kinase by reducing this complex formation. Cdc2 could be dephosphorylated by Cdc25C and become inactive or be phosphorylated by protein kinase, such as Wee1, and then converted into an inactive form. They also suggest that more studies are needed in the future to test it and to define its upstream events in PC3 cells.
Ref : Jun Yan and Aaron E. Katz, Integr Cancer Ther 2010 9: 186
Thursday, October 6, 2011
Blueberry powder may control triple negative breast cancer
Wednesday, November 11, 2009
Anti-aging products from Schisandra Chinesis..


Recently, Glissandra Skincare Inc, announced the launch of three anti-aging products. As per the claim by the company, the key ingredient is Glissandrin,™ an exclusive suite of powerful extracts from the Schisandra berry (see above picture).
In both in-vitro and in-vivo studies, the proprietary Glissandrin formulation has proven effective in improving the visible signs of skin aging. Glissandrin does not change the cells, it nourishes them with a unique combination of natural ingredients and advanced technology, thereby supporting the healthiness of the skin cells and helping to sustain their natural ability to combat the leading causes of skin aging.
Ref : http://www.glissandra.com/story.html
Thursday, February 28, 2013
Positive health indicators associated with avocado consumption
The fruit of horticultural cultivars has a markedly higher fat content than most other fruit, mostly monounsaturated fat, and as such serves as an important staple in the diet of various groups where access to other fatty foods (high-fat meats and fish, dairy products, etc.) is limited.
A ripe avocado yields to gentle pressure when held in the palm of the hand and squeezed. The flesh is prone to enzymatic browning; it turns brown quickly after exposure to air. To prevent this, lime or lemon juice can be added to avocados after they are peeled.
Monday, February 19, 2018
One hundred percent fruit juice does not alter blood sugar levels

Tuesday, April 27, 2010
Chokeberry extract reduces weight gain in insulin-resistant animals.....
Now Drs. Bolin Qin and Richard Anderson from the US Department of Agriculture in Beltsville, MD, have come up with some more interesting info about chokeberries, i.e., "chokeberry extract reduces weight gain in insulin-resistant animals".
Saturday, May 18, 2019
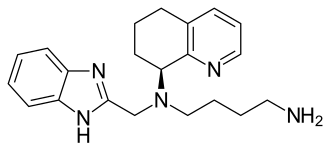
FDA Approves Dovato (dolutegravir/lamivudine) for HIV-1 Infection

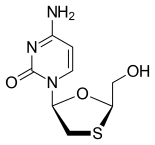
Pedro Cahn, principal investigator for the GEMINI study program said: “People are now living longer with HIV and will spend a lifetime taking drugs to suppress their virus. The approval of the fixed dose combination of dolutegravir and lamivudine, a complete, single-tablet, two-drug regimen, marks a pivotal moment in the treatment of HIV-1. Treatment-naïve people living with the virus have a powerful option that delivers non-inferior efficacy to a dolutegravir-based three-drug regimen, allowing them to take fewer ARVs and get and remain suppressed.”
About Dovato (dolutegravir/lamivudine)
https://en.wikipedia.org/wiki/Lamivudine
Monday, May 7, 2012
Berries, Tea May Cut Men’s Odds for Parkinson’s Disease..
“For total flavonoids, the beneficial result was only in men. But, berries are protective in both men and women,” said the study’s lead author, Dr. Xiang Gao, a research scientist at the Harvard School of Public Health and an associate epidemiologist at Harvard Medical School and Brigham and Women’s Hospital in Boston.
Monday, December 7, 2015
Cranberry juice consumption may protect against cardiovascular disease
Tuesday, September 10, 2024
FDA Approves Xolremdi (mavorixafor) for Use in Patients with WHIM Syndrome
X4 Pharmaceuticals (Nasdaq: XFOR), a company driven to improve the lives of people with rare diseases of the immune system, today announced that the U.S. Food and Drug Administration (FDA) has approved Xolremdi™ (mavorixafor) capsules for use in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes.
Xolremdi, a selective CXC chemokine receptor 4 (CXCR4) antagonist, is the first therapy specifically indicated in patients with WHIM syndrome, a rare, combined primary immunodeficiency and chronic neutropenic disorder caused by CXCR4 pathway dysfunction. People with WHIM syndrome characteristically have low blood levels of neutrophils (neutropenia) and lymphocytes (lymphopenia) and experience serious and/or frequent infections. The FDA granted Breakthrough Therapy Designation to mavorixafor in WHIM syndrome and evaluated the New Drug Application (NDA) under Priority Review, a designation for therapies that have the potential to provide significant improvement in the treatment, diagnosis, or prevention of serious conditions.
“The approval of Xolremdi is a transformational milestone both for X4 and, more importantly, for the WHIM syndrome community,” said Paula Ragan, Ph.D., President and Chief Executive Officer of X4 Pharmaceuticals. “We are incredibly grateful to the people living with WHIM syndrome, their families, and the investigators who took part in our clinical program, to U.S. regulators for their continued focus on rare-disease treatment development, and to our dedicated employees for making this targeted breakthrough therapy a reality.”
“Effective and innovative treatments are critical for those diagnosed with a primary immunodeficiency. The approval of Xolremdi marks an important advancement for people living with WHIM syndrome, who are susceptible to serious and frequent infections,” said Jorey Berry, President and Chief Executive Officer of the Immune Deficiency Foundation (IDF). “We are very pleased to have been a partner to X4 in their journey to bring this much-needed treatment to this underserved rare disease community.”
Teresa K. Tarrant, M.D., Associate Professor of Medicine, Rheumatology, and Immunology at Duke University School of Medicine and a principal investigator in the 4WHIM trial, commented on the news: “Until now, supportive care for people with WHIM syndrome has focused on symptom management and not the underlying cause of disease — the dysfunction of the CXCR4 pathway. I am thrilled that with the approval of Xolremdi, a therapy designed to address dysregulated CXCR4 pathway signaling, we now have a targeted treatment that has demonstrated the ability to elevate absolute neutrophil and lymphocyte counts, increasing WHIM patients’ ability to fight infections.”
The FDA approval of Xolremdi was based on results of the pivotal, 4WHIM Phase 3 clinical trial, a global, randomized, double-blind, placebo-controlled, 52-week multicenter study that evaluated the efficacy and safety of Xolremdi in 31 people aged 12 years and older diagnosed with WHIM syndrome. The efficacy of Xolremdi was determined by improvement in absolute neutrophil counts (ANC), improvement in absolute lymphocyte counts (ALC), and a reduction in infections. In the 4WHIM trial, Xolremdi treatment demonstrated increased time above threshold (≥500 cells/microliter) for absolute neutrophil count (TAT-ANC) vs. placebo (p<0.0001) and increased time above threshold (≥1000 cells/microliter) for absolute lymphocyte count (TAT-ALC) v. placebo (p<0.0001). The efficacy of Xolremdi was further assessed in a composite endpoint consisting of total infection score and total wart change score using a Win-Ratio method. Analyses of the individual components of this composite endpoint showed an approximate 40% reduction in total infection score, weighted by infection severity, in Xolremdi-treated patients compared with placebo-treated patients. There was no difference in total wart change scores between the Xolremdi and placebo treatment arms over the 52-week period. Treatment with Xolremdi also resulted in a 60% reduction in the annualized infection rate compared with placebo-treated patients. The most common adverse reactions reported in the 4WHIM trial (≥10% and more frequently reported than placebo) were: thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
With the FDA approval of Xolremdi, X4 has received a Rare Pediatric Disease Priority Review Voucher that can be used to obtain priority review for a subsequent application or sold to another drug sponsor.