Impax Laboratories, Inc. (NASDAQ: IPXL) today announced that the United States Food and Drug Administration (FDA) has approved the Company's supplemental new drug application (sNDA) for Emverm (mebendazole) 100 mg chewable tablets.
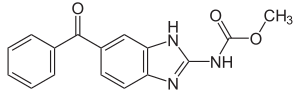
Impax Receives Approval of Emverm (mebendazole) Chewable Tablets

No comments:
Post a Comment