In continuation of my update on oxymetazoline
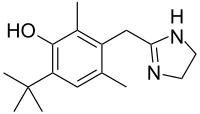
Zoe Diana Draelos, M.D., from Dermatology Consulting Services in High Point, Tenn., and colleagues examined the long-term safety (one year) and efficacy of oxymetazoline cream 1.0 percent in 440 patients with rosacea with moderate-to-severe persistent facial erythema. Assessments were conducted at three and six hours after the dose on day one, and at weeks four, 26, and 52.
The researchers found that 8.2 percent of patients reported treatment-emergent adverse events (TEAEs), the most common were application site dermatitis, paresthesia, pain, and pruritus. The rate of discontinuation mostly due to application-site TEAEs was 3.2 percent. There was no clinically meaningful skin blanching, inflammatory lesions, or telangiectasia. At week 52, a 2-grade or greater composite improvement from baseline in both Clinician Erythema Assessment and Subject Self-Assessment three and six hours after a dose was seen in 36.7 and 43.4 percent of patients, respectively. Following treatment cessation, less than 1 percent of patients experienced a rebound effect.
"This long-term study demonstrated sustained safety, tolerability, and efficacy of oxymetazoline for moderate-to-severe persistent erythema of rosacea," the authors write.Zoe Diana Draelos, M.D., from Dermatology Consulting Services in High Point, Tenn., and colleagues examined the long-term safety (one year) and efficacy of oxymetazoline cream 1.0 percent in 440 patients with rosacea with moderate-to-severe persistent facial erythema. Assessments were conducted at three and six hours after the dose on day one, and at weeks four, 26, and 52.
The researchers found that 8.2 percent of patients reported treatment-emergent adverse events (TEAEs), the most common were application site dermatitis, paresthesia, pain, and pruritus. The rate of discontinuation mostly due to application-site TEAEs was 3.2 percent. There was no clinically meaningful skin blanching, inflammatory lesions, or telangiectasia. At week 52, a 2-grade or greater composite improvement from baseline in both Clinician Erythema Assessment and Subject Self-Assessment three and six hours after a dose was seen in 36.7 and 43.4 percent of patients, respectively. Following treatment cessation, less than 1 percent of patients experienced a rebound effect.
"This long-term study demonstrated sustained safety, tolerability, and efficacy of oxymetazoline for moderate-to-severe persistent erythema of rosacea," the authors write.

No comments:
Post a Comment