TB Alliance’s new drug application (NDA) for the novel tuberculosis (TB) drug candidate pretomanid has been accepted for review by the United States Food and Drug Administration (FDA). The application is for the use of pretomanid as part of a new regimen, in combination with bedaquiline and linezolid, for the treatment of extensively drug-resistant (XDR) TB, treatment intolerant multidrug-resistant (MDR) TB, and treatment non-responsive MDR-TB.
The NDA for pretomanid has been granted Priority Review by FDA. The Prescription Drug User Fee Act (PDUFA) action date for an FDA decision is in third quarter 2019.
TB Alliance will work with manufacturing partners to ensure that pretomanid, if approved for use in the BPaL regimen, will be accessible to those who need it.
About Pretomanid and the BPaL Regimen
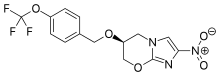
Pretomanid is a new chemical entity and a member of a class of compounds known as nitroimidazooxazines. It has been studied in 20 clinical trials alone or in combination with other anti-TB drugs. Since TB Alliance began development of pretomanid in 2002, it has been administered in a clinical trial setting to more than 1,200 people in 14 countries.
The BPaL regimen (comprised of bedaquiline, pretomanid and linezolid) was first studied clinically in the Phase 3 Nix-TB trial. Nix-TB participants with XDR-TB and treatment intolerant or nonresponsive MDR-TB were enrolled for treatment with the BPaL regimen for six months, extendable to nine months, with the intent to cure. Nix-TB is an open label, single arm trial. According to a modified intention-to-treat analysis of interim results on the first 75 participants presented at the 2018 Union World Conference on Lung Health, 89% of the trial participants had a favorable outcome with their clinical infection resolved and sputum cultures negative for TB after six months of treatment and six months of post-treatment follow-up.
https://www.tballiance.org/portfolio/compound/pretomanid
https://en.wikipedia.org/wiki/Pretomanid
https://www.drugbank.ca/drugs/DB05154

No comments:
Post a Comment