Wednesday, July 31, 2024
Scientists Develop Sensor That Tests Saliva for Breast Cancer
Tuesday, July 30, 2024
Plant-Based Food Intake Linked to Better QoL in Prostate Cancer
Among patients with prostate cancer, greater consumption of plant-based foods is associated with higher scores in quality-of-life domains, according to a study published online Feb. 13 in Cancer.
Stacy Loeb, M.D., Ph.D., from New York University and Manhattan Veterans Affairs in New York City, and colleagues examined the relationship between plant-based diet indices after prostate cancer diagnosis and quality of life in a prospective cohort study involving 3,505 participants in the Health Professionals Follow‐Up Study (1986 to 2016) with nonmetastatic prostate cancer. Overall and healthful plant-based diet indices were calculated using food-frequency questionnaires. The Expanded Prostate Cancer Index Composite was used to calculate quality-of-life scores.
The researchers found that better scores for sexual function, urinary irritation/obstruction, urinary incontinence, and hormonal/vitality were seen in association with a higher plant-based diet index. In the age-adjusted analysis, but not in the multivariable analysis, consuming more healthful plant-based foods was also associated with better sexual and bowel function and improved urinary incontinence and hormonal/vitality scores.
"Individuals with prostate cancer should be advised that incorporating a greater amount of plant‐based foods into their diet could not only reduce the risk of comorbid conditions but also contribute to improved functional outcomes," the authors write.
Ref : https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/cncr.35172
Monday, July 29, 2024
FDA Approves Zepbound (tirzepatide) for Chronic Weight Management
- The U.S. Food and Drug Administration (FDA) approved Eli Lilly and Company's (NYSE: LLY) Zepbound™ (tirzepatide) injection, the first and only obesity treatment of its kind that activates both GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) hormone receptors. Zepbound is indicated for adults with obesity (with a BMI of 30 kg/m2 or greater), or those who are overweight (with a BMI of 27 kg/m2 or greater) and also have weight-related medical problems such as hypertension, dyslipidemia, type 2 diabetes mellitus, obstructive sleep apnea or cardiovascular disease, to lose weight and keep it off. It should be used with a reduced-calorie diet and increased physical activity. Zepbound should not be used with other tirzepatide-containing products or any GLP-1 receptor agonist medicines, and it has not been studied in patients with a history of pancreatitis, or with severe gastrointestinal disease, including severe gastroparesis.
- "Obesity is a chronic disease that can result in serious health complications, including heart disease, stroke and diabetes. Despite our knowledge of obesity as a treatable, chronic disease, people living with obesity still face many challenges in their health and weight management journey," said Joe Nadglowski, president and chief executive officer of the Obesity Action Coalition. "New treatment options bring hope to the many people with obesity who struggle with this disease and are seeking better options for weight management."
- The approval was based on results from the phase 3 SURMOUNT-1 and SURMOUNT-2 trials. In SURMOUNT-1, a study in 2,539 adults with obesity, or excess weight and weight-related medical problems not including diabetes, people taking Zepbound as an adjunct to diet and exercise experienced substantial weight loss compared with placebo at 72 weeks. At the highest dose (15 mg), people taking Zepbound lost on average 48 lb., while at the lowest dose (5 mg), people lost on average 34 lb. (compared to 7 lb. on placebo).
- Additionally, 1 in 3 patients taking Zepbound at the highest dose lost over 58 lb. (25% of body weight), compared to 1.5% on placebo, according to data not controlled for type 1 error. The average starting weight was 231 lb.
- While not approved to treat these conditions, in a clinical trial, people who dieted, exercised and took Zepbound for the treatment of obesity or overweight with weight-related medical problems observed changes in cholesterol and reductions in blood pressure and waist size.
- "Unfortunately, despite scientific evidence to the contrary, obesity is often seen as a lifestyle choice – something that people should manage themselves," said Dr. Leonard Glass, senior vice president global medical affairs, Lilly Diabetes and Obesity. "For decades, diet and exercise have been a go-to, but it's not uncommon for a person to have tried 20-30 times to lose weight with this approach. Research now shows that the body may respond to a calorie-deficit diet by increasing hunger and reducing feelings of fullness, making weight loss more difficult. Lilly is aiming to eliminate misperceptions about this disease and transform how it can be managed."
- Zepbound use may be associated with gastrointestinal adverse reactions, sometimes severe. The most commonly reported adverse events (observed in ≥ 5% of clinical trial participants) were nausea, diarrhea, vomiting, constipation, abdominal pain, dyspepsia, injection-site reactions, fatigue, hypersensitivity reactions, eructation, hair loss and gastroesophageal reflux disease.1 In studies, most nausea, diarrhea and vomiting occurred when people increased their dose – but the effects generally decreased over time. In studies, gastrointestinal side effects were more common in people taking Zepbound than people taking placebo, and people taking Zepbound were more likely than those on placebo to stop treatment because of these side effects. The label for Zepbound includes a Boxed Warning regarding thyroid C-cell tumors. Zepbound is contraindicated in patients with a personal or family history of medullary thyroid carcinoma, in patients with Multiple Endocrine Neoplasia syndrome type 2, and in patients with known serious hypersensitivity to tirzepatide or any of the excipients in Zepbound. See Important Safety Information below and full Prescribing Information and Medication Guide.
- "Far too many hurdles continue to prevent people living with obesity from accessing obesity treatments that could lead to significant weight loss," said Mike Mason, executive vice president and president, Lilly Diabetes and Obesity. "Broader access to these medicines is critical, which is why Lilly is committed to working with healthcare, government and industry partners to ensure people who may benefit from Zepbound can access it."
- Zepbound is expected to be available in the U.S. by the end of the year in six doses (2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg) at a list price of $1,059.87, which is approximately 20% lower than semaglutide 2.4 mg injection for weight loss. List price does not reflect the typical out-of-pocket cost to patients given insurance coverage and discounts. Lilly is putting a commercial savings card program in place that will help people who may benefit from Zepbound better access it.
- People who are commercially insured with coverage for Zepbound may be eligible to pay as low as $25 for a 1-month or 3-month prescription.
- People who are commercially insured without coverage for Zepbound may be eligible to pay as low as $550 for a 1-month prescription of Zepbound, approximately 50% lower than the list price.
- People may begin using the savings card program in the days following product availability at U.S. pharmacies. To learn more about these programs, or to sign up to receive the latest news, please visit www.Zepbound.lilly.com. Terms and conditions apply.
- Tirzepatide is also under regulatory review for weight management in Europe, China, the United Kingdom and several additional markets.
Friday, July 26, 2024
FDA Approves DefenCath (taurolidine and heparin) to Reduce the Incidence of Catheter-Related Bloodstream Infections in Adult Hemodialysis Patients
Joseph Todisco, Chief Executive Officer of CorMedix commented, “The approval of DefenCath marks a major advancement in reducing life-threatening infections for patients receiving hemodialysis via central venous catheters and an important milestone for CorMedix. As the first FDA-approved antimicrobial catheter lock solution designed to prevent CRBSIs, DefenCath offers healthcare providers an option to reduce the risk of infections in a patient population already vulnerable due to underlying kidney failure. We thank all the patients, caregivers, clinical investigators, and our employees who have played an integral role in the development and regulatory approval of DefenCath. Our commercial team along with our broader organization is preparing for commercial launch, and we look forward to working with healthcare providers and facilities to give hemodialysis patients access to DefenCath in early 2024.”
The FDA approval of DefenCath was supported by results from the randomized, double-blind, active control, multicenter pivotal Phase 3 LOCK-IT-100 clinical trial designed to assess the efficacy and safety of DefenCath for reducing the incidence of CRBSIs in patients with kidney failure receiving chronic hemodialysis. In the study, a total of 806 subjects were randomized to receive either DefenCath or heparin as a CLS. Patients in the DefenCath group had a lower incidence of CRBSI events compared to patients in the control group. The Hazard Ratio was 0.29, corresponding to a statistically significant 71% reduction in risk of developing a CRBSI. An independent Data Safety and Monitoring Board recommended an early termination of the study based on demonstrated efficacy and a pre-specified level of statistical significance with no safety concerns. Adverse events were comparable to control.
Edward V. Hickey, III, President of the American Association of Kidney Patients and Chair of the Veterans Health Initiative stated, “Patients and their loved ones have faced many burdens related to kidney failure, including complications caused by catheter related bloodstream infections and associated loss of work, severe disability and death. Until now, patients who need hemodialysis via a central venous catheter have had little choice other than to accept high infection risks associated with the existing standard of care. The FDA’s approval of DefenCath is a meaningful moment for patients and their healthcare providers because they now have a new alternative to reduce the risks of CRBSIs.” Mr. Hickey is a kidney patient, former senior staff member of the U.S. Congress and has served in two presidential administrations.
Thursday, July 25, 2024
FDA Approves Truqap (capivasertib) plus Faslodex for Patients with Advanced HR-Positive Breast Cancer
AstraZeneca’s Truqap (capivasertib) in combination with Faslodex (fulvestrant) has been approved in the US for the treatment of adult patients with hormone receptor (HR)-positive, HER2-negative locally advanced or metastatic breast cancer with one or more biomarker alterations (PIK3CA, AKT1 or PTEN). Eligible patients will have progressed on at least one endocrine-based regimen in the metastatic setting or experienced recurrence on or within 12 months of completing adjuvant therapy.
The approval by the Food and Drug Administration (FDA) was based on the results from the CAPItello-291 Phase III trial published earlier this year in The New England Journal of Medicine.1 In the trial, Truqap in combination with Faslodex reduced the risk of disease progression or death by 50% versus Faslodex alone in patients with tumours harbouring PI3K/AKT pathway biomarker alterations (based on hazard ratio of 0.50, 95% confidence interval 0.38-0.65; p=<0.001; median progression-free survival (PFS) 7.3 versus 3.1 months).
Breast cancer is the most common cancer and one of the leading causes of cancer-related death worldwide.2 HR-positive breast cancer (expressing estrogen or progesterone receptors, or both), is the most common subtype, with more than 65% of tumours considered HR-positive and HER2-low or HER2-negative.3 Collectively, mutations in PIK3CA, AKT1 and alterations in PTEN occur frequently, affecting up to 50% of patients with advanced HR-positive breast cancer.4-6 Endocrine therapies are widely used in this setting, but many patients develop resistance to 1st-line cyclin-dependent kinase (CDK) 4/6 inhibitors and estrogen receptor-targeting therapies, underscoring the need for additional endocrine therapy-based options
Wednesday, July 24, 2024
FDA Approves Ogsiveo (nirogacestat) for Adults with Desmoid Tumors
SpringWorks Therapeutics, Inc. (Nasdaq: SWTX), a commercial-stage biopharmaceutical company focused on severe rare diseases and cancer, announced the U.S. Food and Drug Administration (FDA) approval of Ogsiveo™ (nirogacestat), an oral gamma secretase inhibitor, for the treatment of adult patients with progressing desmoid tumors who require systemic treatment. The FDA previously granted breakthrough therapy, fast track and orphan drug designations to nirogacestat for the treatment of desmoid tumors.
“Our team is honored to deliver the first FDA-approved therapy for patients with desmoid tumors. This community has been waiting for an effective treatment that not only shrinks their tumors but also significantly improves pain, which is the most debilitating symptom reported by people living with desmoid tumors,” said Saqib Islam, Chief Executive Officer of SpringWorks. "We are pleased with the broad label, which includes all progressing adult patients and specifically references improvement in pain, and believe Ogsiveo has the potential to become the new standard of care for people living with these devastating tumors. This is a watershed moment for the desmoid tumor community, and we would like to extend our gratitude to the patients, families, investigators, and advocacy groups involved in the journey to making Ogsiveo available in the U.S.”
Tuesday, July 23, 2024
FDA Approves Fabhalta (iptacopan) for the Treatment of Adults with Paroxysmal Nocturnal Hemoglobinuria (PNH)
Novartis announced the U.S. Food and Drug Administration (FDA) approval of Fabhalta® (iptacopan) as the first oral monotherapy for the treatment of adults with paroxysmal nocturnal hemoglobinuria (PNH)1. Fabhalta is a Factor B inhibitor that acts proximally in the alternative complement pathway of the immune system, providing comprehensive control of red blood cell (RBC) destruction within and outside the blood vessels (intra- and extravascular hemolysis [IVH and EVH]). In clinical trials, treatment with Fabhalta increased hemoglobin levels (≥ 2 g/dL from baseline in the absence of RBC transfusions) in the majority of patients and in APPLY-PNH nearly all patients treated with Fabhalta did not receive blood transfusions.
“An efficacious oral treatment with a demonstrated safety profile could be practice-changing for physicians and help relieve burdens experienced by people with PNH,” said Vinod Pullarkat, MD, MRCP, Clinical Professor, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope. “In clinical studies, iptacopan was superior to anti-C5s in hemoglobin improvement in the absence of RBC transfusion and transfusion avoidance rate, and also effective in complement inhibitor-naïve individuals, by providing clinically meaningful hemoglobin-level increases without the need for blood transfusions.”
The FDA approval is based on the Phase III APPLY-PNH trial in patients with residual anemia (hemoglobin < 10 g/dL) despite prior anti-C5 treatment who switched to Fabhalta, which demonstrated superiority in hemoglobin improvement in the absence of RBC transfusions and in transfusion avoidance rate over patients who stayed on anti-C5 treatments1,2. Approval was also supported by the Phase III APPOINT-PNH study in complement inhibitor-naïve patients1,3. The 24-week core treatment periods in APPLY-PNH and APPOINT-PNH trials respectively showed
More atMonday, July 22, 2024
FDA Approves iDose TR (travoprost intracameral implant) for the Treatment of Glaucoma
Glaukos Corporation (NYSE: GKOS), an ophthalmic medical technology and pharmaceutical company focused on novel therapies for the treatment of glaucoma, corneal disorders and retinal diseases, announced the U.S. Food and Drug Administration (FDA) approval of its New Drug Application (NDA) for a single administration per eye of iDose® TR (travoprost intracameral implant) 75 mcg, a prostaglandin analog indicated for the reduction of intraocular pressure (IOP) in patients with ocular hypertension (OHT) or open-angle glaucoma (OAG).
“With the next generation of procedural pharmaceutical solutions for glaucoma such as iDose TR, we now have a new tool that will confront the standard legacy practice of relying on topical drops, which are known to cause uncomfortable side effects and present a myriad of challenges such as treatment adherence, complex dosing regimens, and difficulty with self-administration,” said John Berdahl, MD, clinician and researcher at Vance Thompson Vision. “The clinical data suggest that iDoseTR is not only effective with a favorable safety profile, but it has potential to relieve patients from the burdens of prescription eye drops for an extended period of time. I look forward to adding this novel therapy into my treatment toolbox for the benefit of my patients.”
The FDA approval is based on results from two prospective, randomized, multicenter, double-masked, Phase 3 pivotal trials (GC-010 and GC-012) designed to compare the safety and efficacy of a single administration of one of two iDose TR models with different travoprost release rates (referred to as the fast- and slow-release iDose TR models, respectively) to topical timolol ophthalmic solution, 0.5% BID (twice a day), in reducing IOP in subjects with open-angle glaucoma or ocular hypertension. In total, the Phase 3 trials randomized 1,150 subjects across 89 clinical sites. The FDA approval and Phase 3 data referenced below is for the slow-release iDose TR model, consistent with the company’s NDA submission and commercialization plans.
Both Phase 3 trials successfully achieved the pre-specified primary efficacy endpoints through 3 months and demonstrated a favorable tolerability and safety profile through 12 months. IOP reductions from baseline over the first 3 months were 6.6-8.4 mmHg in the iDose TR arm, versus 6.5-7.7 mmHg in the timolol control arm (mmHg range represents IOP reduction means across the six U.S. FDA pre-specified timepoints of 8 a.m. and 10 a.m. at Day 10, Week 6 and Month 3). Based on these outcomes, the FDA concluded in the prescribing information that iDose TR demonstrated non-inferiority to timolol ophthalmic solution in IOP reduction during the first 3 months. The FDA also noted that subsequently iDose TR did not demonstrate non-inferiority over the next 9 months.
iDose TR is a first-of-its-kind, long-duration, intracameral procedural pharmaceutical therapy designed to continuously deliver 24/7 therapeutic levels of a proprietary formulation of travoprost inside the eye for extended periods of time. iDose TR is intended to improve the standard of care by addressing the ubiquitous patient non-compliance issues and chronic side effects associated with topical glaucoma medications.
“The FDA approval of iDose TR represents a significant milestone for Glaukos following an extensive pioneering journey since the inception of the original idea nearly 15 years ago. Today’s approval ushers in a new era of interventional glaucoma therapy by enabling a more proactive and reliable approach for patients in need,” said Thomas Burns, Glaukos chairman and chief executive officer. “We believe iDose TR can be a transformative, novel technology able to fundamentally improve the treatment paradigm for patients with open-angle glaucoma or ocular hypertension. We are grateful to the clinical investigators and study participants in the clinical trials for their instrumental roles in helping us reach this important advancement for glaucoma patient care. At Glaukos, we are relentlessly focused on delivering novel therapies for chronic eye diseases and now iDoseTR has the potential to redefine the standard of care for patients in the U.S. affected by open-angle glaucoma and ocular hypertension.”
Friday, July 19, 2024
FDA Approves Iwilfin (eflornithine) as Maintenance Therapy for High-Risk Neuroblastoma
USWM, LLC announced the U.S. Food and Drug Administration (FDA) approval of Iwilfin ™ (eflornithine) 192 mg tablets, a groundbreaking oral maintenance therapy for high-risk neuroblastoma. Iwilfin is indicated to reduce the risk of relapse in adult and pediatric patients with high-risk neuroblastoma who have demonstrated at least a partial response to prior multiagent, multimodality therapy, including anti-GD2 immunotherapy.
Acc
ording to the American Cancer Society, 700-800 cases of neuroblastoma are diagnosed in the U.S. each year, with 90% of diagnoses coming before age 5. Over 50% of these cases are classified as high-risk. High-risk neuroblastoma is a challenging disease, with a high mortality rate driven primarily by the risk of relapse after achieving remission. Approximately half of children with high-risk neuroblastoma do not survive beyond five years from diagnosis. Although existing treatments are effective in helping patients achieve remission, patients lack options to sustain it. Avoiding relapse is crucial to improving survival rates.
“We are thrilled to announce the FDA approval of Iwilfin , which provides a new and much-needed treatment option for children with high-risk neuroblastoma,” said Breck Jones, Chief Executive Officer of US WorldMeds. “The goal for treating these young patients is to prevent relapse, and advancing therapeutic options is critical to this mission. Iwilfin offers new hope and improved outcomes for these vulnerable children.”
Iwilfin is taken orally, with or without food, twice daily for two years. Iwilfin is generally well-tolerated, with side effects typically manageable through dose modifications. The most common side effects are hearing loss, otitis media, pyrexia, pneumonia, and diarrhea. Important Safety Information can be found below.
US WorldMeds partnered with the Beat Childhood Cancer Research Consortium at Penn State University, which conducted the preclinical and clinical research to help advance this vital therapy. The Consortium represents a group of over 50 hospitals that offer collaboration through a network of childhood cancer clinical trials.
“Our partnerships were instrumental in bringing Iwilfin through the FDA registration process,” said Kristen Gullo, Vice President of Development and Regulatory Affairs at US WorldMeds. “We are thankful for the dedication of our partners, specifically the Beat Childhood Cancer Research Consortium, who work tirelessly to improve treatment outcomes for pediatric cancer patients. This FDA approval represents a beacon of hope for the high-risk neuroblastoma community and a significant step forward in the fight against this devastating disease.”
REF ; https://en.wikipedia.org/wiki/Eflornithine
Thursday, July 18, 2024
FDA Approves Wainua (eplontersen) for the Treatment of Adults with Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis
"Many people living with hereditary transthyretin-mediated amyloid polyneuropathy are unable to fully enjoy their lives because of the relentless, progressive and debilitating effects of the disease," said Michael J. Polydefkis, M.D., professor of neurology at Johns Hopkins University School of Medicine and an investigator in the NEURO-TTRansform study. "Approval of Wainua represents a meaningful advancement in treatment, one that gives those who are living with transthyretin-mediated amyloid polyneuropathy help managing the disease."
ATTRv-PN is a debilitating disease that leads to peripheral nerve damage with motor disability within five years of diagnosis and, without treatment, is generally fatal within a decade. Wainua is a ligand-conjugated antisense oligonucleotide (LICA) medicine designed to reduce the production of TTR protein at its source.
"The FDA approval of Wainua marks an important milestone for people living with hereditary transthyretin-mediated amyloid polyneuropathy, who will now have an effective, well-tolerated treatment that can be self-administered via auto-injector to combat this devastating disease," said Brett P. Monia, Ph.D., chief executive officer at Ionis. "It is also a pivotal moment for Ionis as Wainua will be the first in a steady cadence of potential commercial launches for the company. We are proud to have discovered and, together with AstraZeneca, developed Wainua, and are grateful to the patients, caregivers and investigators who participated in our clinical studies, as well as for the dedication of our scientists and researchers."
"People with hereditary transthyretin-mediated amyloid polyneuropathy, and other forms of amyloidosis, are often misdiagnosed since symptoms can mirror other conditions," said Isabelle Lousada, President and CEO, Amyloidosis Research Consortium "The path to getting an accurate diagnosis can often be a long, arduous journey and it is critical that a timely and accurate diagnosis is made not only for the individual experiencing symptoms but for their families and loved ones. It is exciting to see new innovations coming through and increased efforts to raise awareness in an area that has often been overlooked or neglected."
Wednesday, July 17, 2024
FDA Approves Zelsuvmi (berdazimer topical gel) for the Treatment of Molluscum Contagiosum
Tuesday, July 16, 2024
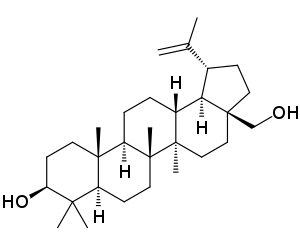
FDA Approves Filsuvez (birch triterpenes) Topical Gel for the Treatment of Epidermolysis Bullosa
FDA Approves Filsuvez () Topical Gel for the Treatment of Epidermolysis Bullosa
EB is a debilitating inherited skin disease that causes a person’s skin to be so fragile it can be injured just from touch. This rare, chronic, and distressing disorder affects infants, children and adults and is intensely painful; recurrent blistering and chronic wounds can result in intolerable pain with limited mobility. Living with EB entails daily challenges to navigate, including slow-healing wounds at risk of infection and painful dressing changes.