Ipsen (Euronext: IPN; ADR: IPSEY) today announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Iqirvo (elafibranor) 80 mg tablets for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults who have an inadequate response to UDCA, or as monotherapy in patients unable to tolerate UDCA. Iqirvo may be prescribed immediately in the U.S. for eligible patients.
This indication is approved under accelerated approval based on the reduction of alkaline phosphatase (ALP). Improvement in survival or prevention of liver decompensation events has not been demonstrated. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). Iqirvo is not recommended for people who have or develop decompensated cirrhosis (e.g., ascites, variceal bleeding, hepatic encephalopathy).
“For a significant number of people living with PBC, available treatments do not control the condition and may exacerbate symptoms of PBC. Left unmanaged, PBC can progress, leading to liver failure and in some cases, the need for a liver transplant,” said Christelle Huguet, Executive Vice President, Head of Research and Development at Ipsen. “Iqirvo demonstrated statistically significant improvements in biochemical response compared to UDCA alone. Iqirvo is, therefore a much-needed treatment option and the first new medicine for PBC in nearly a decade.”
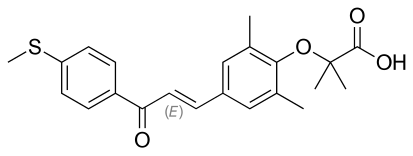
Iqirvo is a first-in-class oral, once-daily peroxisome proliferator-activated receptor (PPAR) agonist. Iqirvo was in-licensed from GENFIT in 2021. The accelerated approval of Iqirvo is based on data from the Phase III ELATIVE trial published in the New England Journal of Medicine1. The ELATIVE trial demonstrated that 13 times more patients achieved the composite primary endpoint of biochemical response when treated with Iqirvo plus UDCA (n=108) versus placebo plus UDCA (=53) (respectively 51% versus 4% for a 47% treatment difference). ALP is a biochemical marker and is used as a surrogate endpoint in PBC trials. Secondary endpoints showed normalization in ALP levels in only Iqirvo-treated patients (15% for Iqirvo plus UDCA (n=108) versus 0% for placebo plus UDCA (n=53)). Most patients (95%) received study treatment (Iqirvo or placebo) in combination with UDCA.
The most common adverse reactions with Iqirvo reported in ≥10% of study participants were weight gain, abdominal pain, diarrhea, nausea, and vomiting. Some study participants treated with Iqirvo experienced myalgia, myopathy and rhabdomyolysis; fractures; adverse effects on fetal and newborn development; drug-induced liver injury; hypersensitivity reactions; or biliary obstruction. See full Important Safety Information below.
“Data from the pivotal Phase III ELATIVE clinical trial demonstrated that Iqirvo is an effective second-line treatment for patients with PBC with favorable benefit and risk data,” said Dr. Kris Kowdley, Director at Liver Institute Northwest, Washington, and a primary investigator on the ELATIVE study. “The approval of Iqirvo will allow healthcare providers in the U.S. to address an unmet need with the potential to significantly reduce ALP levels for our patients with PBC.”
PBC is a rare, autoimmune, cholestatic liver disease where a build-up of bile and toxins (cholestasis) and chronic inflammation causes irreversible fibrosis (scarring) of the liver and destruction of the bile ducts. Impacting approximately 100,000 people in the U.S.2, the majority being women, PBC is a lifelong condition that can worsen over time if not effectively treated, leading to liver transplant and in some cases, premature death. PBC also affects day-to-day life, with people most commonly experiencing severe fatigue symptoms and debilitating itch (pruritus).
“People living with PBC can feel like the symptoms they experience are dismissed by family members, friends, or even their doctors because they have not experienced something similarly disruptive in their lives. People with PBC may also feel uncertainty around the disease progression and if, or when, their liver health may deteriorate,” said Carol Roberts, Executive President of PBCers, a patient advocacy organization in the U.S. providing support to people living with PBC. “Earlier diagnosis and education about PBC, along with new treatment options are important to meet the current needs of people living with PBC.”
Iqirvo has been submitted to the European Medicines Agency (EMA) and the UK Medicines and Healthcare Products Regulatory Agency (MHRA), seeking authorization for PBC, with final EMA and MHRA regulatory decisions anticipated in the second half of 2024. The FDA approval of Iqirvo further strengthens Ipsen’s portfolio of treatments for rare cholestatic liver diseases available to patients in the U.S. This includes our FDA-approved medicine for the treatment of pruritus in patients three months and older with progressive familial intrahepatic cholestasis (PFIC) and for the treatment of cholestatic pruritus in patients from 12 months of age with Alagille syndrome (ALGS).