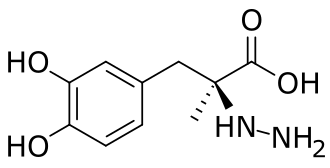
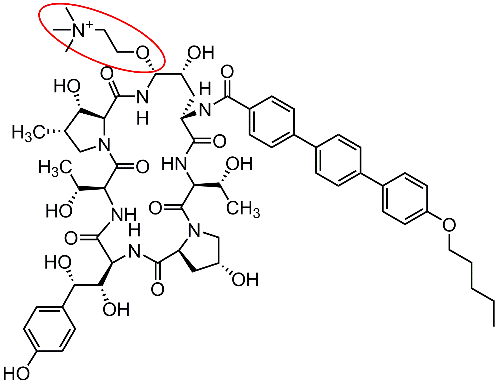
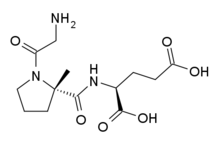
Friday, August 16, 2024
Zevra Therapeutics Announces Resubmission of Arimoclomol New Drug Application to the U.S. Food and Drug Administration
Thursday, August 15, 2024
Zealand Pharma Submits New Drug Application to the US FDA for Glepaglutide in Short Bowel Syndrome
“Short bowel syndrome with intestinal failure is a complex, chronic and severe condition in which individuals are dependent on receiving fluids and nutrition parenterally. While life-sustaining, parenteral support poses significant restrictions on daily life and carries a risk of serious and life-threatening complications. More effective and convenient treatments to further reduce parenteral support are needed, with the ultimate goal of discontinuing parenteral support and achieving enteral autonomy,” said David Kendall, MD, Chief Medical Officer of Zealand Pharma. “We believe glepaglutide, once approved, can reduce both the burden of parenteral support and of daily dosing of existing GLP-2 treatment for people living with SBS and intestinal failure, and we are pleased to submit this treatment for regulatory review and potential approval in the US.”
Wednesday, August 14, 2024
Journey Medical Corporation Submits New Drug Application to FDA for DFD-29 to Treat Rosacea
“This NDA submission is a significant milestone for Journey Medical and we look forward to collaborating with the FDA during its review to bring DFD-29, a potentially differentiated, best-in-class oral rosacea treatment, one step closer to patients. Based on the data seen in our pivotal trials, DFD-29 could fundamentally improve the treatment paradigm for patients suffering from both inflammatory lesions and erythema (redness) from rosacea,” said Claude Maraoui, Co-Founder, President and Chief Executive Officer of Journey Medical.
Friday, August 9, 2024
Amneal Announces Complete Response Resubmission for IPX203 New Drug Application
“We are pleased to provide our complete response resubmission for IPX203 as we look to expand our Parkinson’s franchise,” said Chirag and Chintu Patel, Co-Chief Executive Officers at Amneal. “We look forward to launching this much-needed treatment in the second half of 2024, subject to FDA approval.”
PD is characterized by slowness of movement, stiffness, resting tremor and impaired balance. While PD is not considered a fatal disease, it is associated with significant morbidity and disability. The average age at diagnosis for patients with PD is 60; as people live longer, the number of patients living with PD is predicted to grow significantly over the coming decades.
Thursday, August 8, 2024
Defender Pharmaceuticals Receives Complete Response Letter from the U.S. Food and Drug Administration for its Intranasal Scopolamine (DPI-386) New Drug Application for the Prevention of Nausea and Vomiting Induced by Motion in Adults
Certain motions cause discomfort in individuals while engaged in various leisure or travel-related activities. Most forms of travel, whether on land, in the air, or on the water, can trigger symptoms such as nausea and vomiting (example: flying, boating/fishing, car, bus, and train). Symptoms induced by motion can also have a detrimental impact on the ability of various military personnel and astronauts to perform assigned duties, potentially impacting readiness and negatively impacting resources. Motion-related discomfort is a common and transient response to unfamiliar or unnatural motion or contradictory spatial sensory information, resulting in decrements to performance of tasks, pallor, cold sweating, nausea and vomiting. Prolonged exposure to certain motions may induce sopite-related symptoms such as loss of drive and concentration, drowsiness, sleepiness, apathy, depression, and a feeling of impending doom.
Wednesday, August 7, 2024
Lykos Therapeutics Announces FDA Acceptance and Priority Review of New Drug Application for MDMA-Assisted Therapy for PTSD
Lykos, with longstanding roots in advocacy for psychedelic medicine, pioneered the first randomized, double-blind, placebo controlled clinical trials evaluating the efficacy and safety of MDMA-assisted therapy as an investigational modality using midomafetamine (MDMA) in combination with psychological intervention to treat PTSD.
With a growing body of evidence supporting the potential medical use of MDMA, in 2017 the FDA granted the company's investigational MDMA-assisted therapy Breakthrough Therapy designation, a process designed to expedite the development and review of drugs intended to treat serious conditions for which preliminary scientific evidence indicates that it may demonstrate a substantial improvement over available therapies. If approved by the FDA, the U.S. Drug Enforcement Administration ("DEA") would be required to reschedule MDMA making it available for prescription medical use.
Monday, August 5, 2024
Liquidia Corporation Provides Update on New Drug Application for Yutrepia (treprostinil) inhalation powder
Monday, October 10, 2022
Cidara Therapeutics Announces FDA Acceptance for Priority Review of New Drug Application for Rezafungin for the Treatment of Candidemia and Invasive Candidiasis
Cidara Therapeutics, Inc. , a biotechnology company developing long-acting therapeutics designed to improve the standard of care for patients facing serious diseases, announced the FDA acceptance for filing and granted Priority Review to its New Drug Application (NDA) for rezafungin for the treatment of candidemia and invasive candidiasis. The FDA has assigned a Prescription Drug User Fee Act (PDUFA) target action date of March 22, 2023 enabled by rezafungin’s designation as a Qualified Infectious Disease Product (QIDP) and has indicated that it is currently planning to hold an advisory committee meeting to discuss the application. QIDP designation is reserved for antibacterial and antifungal drug candidates intended to treat serious or life-threatening infections. Rezafungin is a novel, once-weekly echinocandin antifungal being developed for the treatment of candidemia and invasive candidiasis, as well as for the prophylaxis of invasive fungal infections in patients undergoing allogeneic blood and marrow transplant.
“Today’s announcement is an important step forward for patients fighting difficult-to-treat and often deadly candidemia and invasive candidiasis, and represents a critical milestone for Cidara’s rezafungin development program,” said Jeff Stein, President, and CEO of Cidara Therapeutics. “The data generated across our Phase 2 and Phase 3 trials demonstrated that rezafungin could transform the current standard of care for the treatment of invasive Candida infections, and we are excited that rezafungin could potentially be the first new drug approved for this indication in over a decade.”
The NDA submission for rezafungin was supported by positive clinical data from the global ReSTORE Phase 3 and STRIVE Phase 2 clinical trials. Rezafungin dosed once-weekly demonstrated statistical non-inferiority versus caspofungin, the current standard of care, dosed once-daily, meeting the primary endpoints for both the FDA and the European Medicines Agency (EMA).
Cidara retains the rights to rezafungin in Japan and has licensed the commercial rights to Melinta Therapeutics in the U.S. and Mundipharma Medical in all other geographies.
Monday, September 12, 2022
Acadia Pharmaceuticals Announces Trofinetide New Drug Application for the Treatment of Rett Syndrome has been Accepted for Filing and Review by U.S. FDA
Acadia Pharmaceuticals Inc. announced the U.S. Food and Drug Administration (FDA) acceptance for filing its New Drug Application (NDA) of trofinetide for the treatment of Rett syndrome. The FDA has granted a priority review and assigned a PDUFA (Prescription Drug User Fee Act) action date of March 12, 2023. The FDA has also informed the company that at this time they are not planning to hold an Advisory Committee meeting.
“We’re pleased that the FDA has accepted our NDA filing and we will be working closely with them to facilitate completion of the review in a timely manner,” said Steve Davis, Acadia’s Chief Executive Officer. “If approved, trofinetide will be the first drug available for the treatment of Rett syndrome, a rare and devastating condition for patients and their families. This milestone reinforces Acadia’s ongoing commitment to advancing research into high unmet needs in disorders affecting the central nervous system.”
Rett syndrome is a complex, multisystem neurodevelopmental disorder that includes a period of normal development followed by significant developmental regression with loss of language and hand function skills, impaired gait and development of hand stereotypes.1,2 It occurs worldwide in approximately one of every 10,000 to 15,000 female births.3
“Rett is a complex disease that can present with a diverse array of symptoms. In clinical trials, trofinetide demonstrated a significant improvement in a range of Rett syndrome symptoms,” said Jeffrey L. Neul, M.D., Ph.D., Annette Schaffer Eskind Chair and Director, Vanderbilt Kennedy Center, Professor of Pediatrics, Division of Neurology, Pharmacology, and Special Education, Vanderbilt University Medical Center and Phase 3 Lavender™ study investigator. “We look forward to the FDA’s review of this submission and the prospect of having access to the first approved treatment for Rett syndrome.”
The NDA is supported by results from the pivotal Phase 3 Lavender study evaluating the efficacy and safety of trofinetide versus placebo in 187 girls and young women aged 5-20 years with Rett syndrome. The study demonstrated a statistically significant improvement over placebo on the co-primary endpoints, the Rett Syndrome Behaviour Questionnaire (RSBQ) total score change from baseline to 12 weeks (p=0.0175; effect size=0.37) and the Clinical Global Impression-Improvement (CGI-I) scale score (p=0.0030; effect size=0.47). The RSBQ is a caregiver assessment of the core symptoms of Rett syndrome, and the CGI-I is a global physician assessment of worsening or improving of Rett syndrome. In addition, the study also met its key secondary endpoint, the Communication and Symbolic Behavior Scales Developmental Profile™ Infant-Toddler Checklist–Social Composite Score (CSBS-DP-IT–Social) change from baseline to week 12 (p=0.0064; effect size=0.43), a caregiver assessment of ability to communicate.
In 2018, Acadia entered into an exclusive license agreement with Neuren Pharmaceuticals Limited (ASX: NEU) for the development and commercialization of trofinetide for the treatment of Rett syndrome and other indications in North America. In addition to receiving priority review by the FDA, trofinetide has been granted Fast Track Status and Orphan Drug Designation for the treatment of Rett syndrome in the U.S. and has been granted Rare Pediatric Disease (RPD) designation by the FDA. Upon FDA approval of a product with RPD designation, the sponsor can receive a Priority Review Voucher, which can be used to obtain priority review for a subsequent application.
Thursday, January 27, 2022
Spectrum Pharmaceuticals Submits New Drug Application for Poziotinib
Spectrum Pharmaceuticals, a biopharmaceutical company focused on novel and targeted oncology therapies, announced the submission of its New Drug Application (NDA) for poziotinib to the U.S. Food and Drug Administration (FDA) for use in patients with previously treated locally advanced or metastatic non-small cell lung cancer (NSCLC) with HER2 exon 20 insertion mutations. The NDA submission is based on the positive results of Cohort 2 from the ZENITH20 clinical trial, which assessed the safety and efficacy of poziotinib. The product has received Fast Track designation and there is currently no treatment specifically approved by the FDA for this indication.
“The NDA submission for poziotinib marks an important step in achieving a first treatment for patients with HER2 exon 20 insertion mutations in lung cancer,” said Joe Turgeon, President and CEO of Spectrum Pharmaceuticals. “I want to thank the patients, investigators and our internal staff who have passionately worked to achieve this important milestone in an area of high unmet medical need.”
ZENITH20 Cohort 2 Clinical Results Summary
Results for Cohort 2 of the ZENITH20 clinical trial have been published in the Journal of Clinical Oncology (November 29, 2021), and can be accessed by clicking here.
Cohort 2 enrolled 90 patients who received an oral once daily dose of 16 mg of poziotinib. The intent-to-treat analysis demonstrated a confirmed objective response rate (ORR) of 27.8% (95% Confidence Interval (CI), 18.9%-38.2%). The observed lower bound of 18.9% exceeded the pre-specified lower bound of 17%. The median duration of response was 5.1 months and the median progression free survival was 5.5 months. In this cohort, 87% of patients had drug interruptions with 11 patients (12%) permanently discontinuing due to adverse events. 13 patients (14%) had treatment-related serious adverse events. As previously announced, the company had a successful pre-NDA meeting with the FDA which resulted in an agreement to submit an NDA for poziotinib. During the meeting, Spectrum confirmed with the FDA that Cohort 2 data could serve as the basis of an NDA submission. The company will continue to work with the FDA as appropriate, while the agency conducts its review.
More : https://investor.sppirx.com/news-releases/news-release-details/spectrum-pharmaceuticals-submits-new-drug-application-poziotinib
https://en.wikipedia.org/wiki/Poziotinib
Friday, January 7, 2022
New Drug Application for Tebipenem HBr for the Treatment of Complicated Urinary Tract Infections including Pyelonephritis
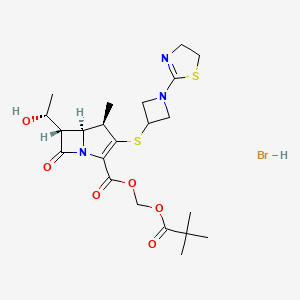
Spero Therapeutics, Inc. announced the submission of a new drug application (NDA) to the U.S. Food and Drug Administration (FDA), seeking approval for tebipenem HBr tablets for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, caused by susceptible microorganisms. If approved, tebipenem HBr would be the only oral carbapenem antibiotic available for use in cUTI.
“With the submission of this NDA, we have taken a major step towards potentially providing a substantial number of appropriate cUTI patients with an oral treatment option that could replace historical use of intravenous (IV) therapy,” said Ankit Mahadevia, M.D., Chief Executive Officer of Spero Therapeutics. “If approved, we believe tebipenem HBr could help patients significantly, and the avoidance of IV administration could lead to reduced healthcare resource utilization. We look forward to working with the FDA during the NDA review process as we prepare for tebipenem HBr’s anticipated launch in the second half of 2022.”
The NDA submission includes previously communicated positive data from the Phase 3 ADAPT-PO trial. This data showed that ADAPT-PO met its primary endpoint by demonstrating that oral tebipenem HBr was statistically non-inferior to IV ertapenem in the treatment of patients with cUTI and patients with acute pyelonephritis (AP).
https://pubchem.ncbi.nlm.nih.gov/compound/Tebipenem-pivoxil-hydrobromide
https://en.wikipedia.org/wiki/Tebipenem
Thursday, January 6, 2022
Spectrum Pharmaceuticals Submits New Drug Application for Poziotinib for metastatic NSCLC with HER2 exon 20 insertion mutations.

Spectrum Pharmaceuticals, a biopharmaceutical company focused on novel and targeted oncology therapies, announced that it has submitted its New Drug Application (NDA) for poziotinib to the U.S. Food and Drug Administration (FDA) for use in patients with previously treated locally advanced or metastatic non-small cell lung cancer (NSCLC) with HER2 exon 20 insertion mutations. The NDA submission is based on the positive results of Cohort 2 from the ZENITH20 clinical trial, which assessed the safety and efficacy of poziotinib. The product has received Fast Track designation and there is currently no treatment specifically approved by the FDA for this indication.
“The NDA submission for poziotinib marks an important step in achieving a first treatment for patients with HER2 exon 20 insertion mutations in lung cancer,” said Joe Turgeon, President and CEO of Spectrum Pharmaceuticals. “I want to thank the patients, investigators and our internal staff who have passionately worked to achieve this important milestone in an area of high unmet medical need.”
ZENITH20 Cohort 2 Clinical Results Summary
Results for Cohort 2 of the ZENITH20 clinical trial have been published in the Journal of Clinical Oncology (November 29, 2021), and can be accessed by clicking here.
Cohort 2 enrolled 90 patients who received an oral once daily dose of 16 mg of poziotinib. The intent-to-treat analysis demonstrated a confirmed objective response rate (ORR) of 27.8% (95% Confidence Interval (CI), 18.9%-38.2%). The observed lower bound of 18.9% exceeded the pre-specified lower bound of 17%. The median duration of response was 5.1 months and the median progression free survival was 5.5 months. In this cohort, 87% of patients had drug interruptions with 11 patients (12%) permanently discontinuing due to adverse events. 13 patients (14%) had treatment-related serious adverse events. As previously announced, the company had a successful pre-NDA meeting with the FDA which resulted in an agreement to submit an NDA for poziotinib. During the meeting, Spectrum confirmed with the FDA that Cohort 2 data could serve as the basis of an NDA submission. The company will continue to work with the FDA as appropriate, while the agency conducts its review.
About the ZENITH20 Clinical Trial
The ZENITH20 study consists of seven cohorts of NSCLC patients. Cohorts 1 (EGFR) and 2 (HER2) in previously treated NSCLC patients with exon 20 mutations and Cohort 3 (EGFR) in first-line patients have completed enrollment. Cohort 4 (HER2) in first-line NSCLC patients with exon 20 mutations is still enrolling patients. Cohorts 1- 4 are each independently powered for a pre-specified statistical hypothesis and the primary endpoint is objective response rate (ORR). Cohort 5 includes previously treated or treatment-naïve NSCLC patients with EGFR or HER2 exon 20 insertion mutations. Cohort 6 includes NSCLC patients with classical EGFR mutations who progressed while on treatment with first-line osimertinib and developed an additional EGFR mutation. Cohort 7 includes NSCLC patients with a variety of less common mutations in EGFR or HER2 exons 18-21 or the extracellular or transmembrane domains
https://en.wikipedia.org/wiki/Poziotinib
Wednesday, January 5, 2022
Stealth BioTherapeutics Submits Elamipretide New Drug Application to FDA for Treatment of Barth Syndrome
Stealth BioTherapeutics Corp, announced the submission of a New Drug Application (NDA) to the U.S. Food and Drug Adminstration (FDA) for elamipretide, the company's lead product candidate, for the treatment of Barth syndrome, an ultra-rare genetic condition with no FDA- or EMA-approved therapies.

"Children and young adults affected by Barth syndrome are suffering from life limiting, progressive cardiomyopathy, exercise intolerance, and debilitating fatigue for which there are no approved treatment options," said Chief Executive Officer Reenie McCarthy. "We initiated our Barth syndrome development efforts at the request of the Barth syndrome community. We respect the patient community's perspective regarding its tolerance for some uncertainty of benefit in considering therapies for this ultra rare disease, and with our NDA submission, have answered its petition that we submit our application. We know that the FDA has similarly heard the voice of these patients, and we look forward to continued dialogue with the FDA as it evaluates our submission for filing and review."
The NDA submission is based on results from the SPIBA-001 Phase 3 Retrospective Natural History Control Trial, which compared data from the open-label portion of the TAZPOWER Phase 2/3 clinical trial to matched natural history controls. SPIBA-001 met its primary and most secondary endpoints, demonstrating elamipretide-mediated improvements in assessments of exercise tolerance, strength and cardiac function that are unexpected in the natural course of this progressively debilitating disease. In addition, improvements were observed during the TAZPOWER Phase 2/3 clinical trial and open label extension in several surrogate and intermediate clinical endpoints that may be reasonably likely to predict clinical benefit for patients suffering from this serious disease, potentially supporting an accelerated approval pathway. Although the FDA has recommended that additional controlled data be generated to support NDA review, neither the FDA nor the Company has identified a feasible trial design due to the ultra-rare nature of this disease. In light of FDA's view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review, there is no assurance that the FDA will file the NDA. Stealth believes, however, that the data could support an NDA review, and has accordingly submitted the NDA as requested by the Barth syndrome patient community.
Elamipretide was previously granted rare pediatric designation, fast track designation, and orphan drug designation by the FDA, and orphan drug designation by the EMA, for the treatment of Barth Syndrome.
About Barth Syndrome
Barth syndrome is an ultra-rare genetic condition characterized by cardiac abnormalities often leading to heart failure and reduced life expectancy, recurrent infections, muscle weakness and delayed growth. Barth syndrome occurs almost exclusively in males and is estimated to affect one in 200,000 to 400,000 individuals worldwide. There are currently no FDA- or EMA-approved therapies for patients with Barth syndrome.
https://en.wikipedia.org/wiki/Elamipretide
Tuesday, January 4, 2022
Axsome Therapeutics Announces FDA Acceptance of New Drug Application for AXS-07 (meloxicam-rizatriptan) for the Acute Treatment of Migraine
In continuation of my update on meloxicam,
Axsome Therapeutics, Inc. that the U.S. Food and Drug Administration (FDA) has accepted for filing the Company’s New Drug Application (NDA) for AXS-07 for the acute treatment of migraine, and has set a Prescription Drug User Fee Act (PDUFA) target action date of April 30, 2022 for the NDA. AXS-07 (MoSEIC™ meloxicam-rizatriptan) is a novel, oral, rapidly absorbed, multi-mechanistic, investigational medicine for migraine.
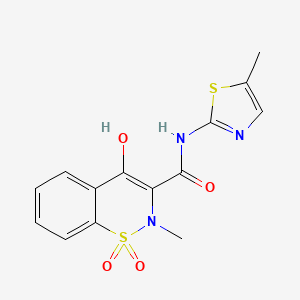
meloxicam
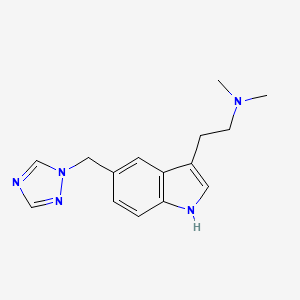
Rizatriptan
“The FDA’s acceptance of the NDA for AXS-07 is an important milestone for Axsome as it brings us closer to potentially making this multi-mechanistic treatment available to migraine patients in need,” said Herriot Tabuteau, MD, Chief Executive Officer of Axsome. “We look forward to continued interactions with the FDA during the review process.”
The NDA is supported by results from two Phase 3 randomized, double-blind, controlled trials of AXS-07 in the acute treatment of migraine, the MOMENTUM and INTERCEPT trials, which demonstrated statistically significant elimination of migraine pain with AXS-07 compared to placebo and active controls.
AXS-07 is a novel, oral, rapidly absorbed, multi-mechanistic investigational medicine for the acute treatment of migraine, consisting of MoSEIC™ meloxicam and rizatriptan. Meloxicam is a new molecular entity for migraine enabled by Axsome’s MoSEIC (Molecular Solubility Enhanced Inclusion Complex) technology, which results in rapid absorption of meloxicam while maintaining a long plasma half-life. Meloxicam is a COX-2 preferential non-steroidal anti-inflammatory drug and rizatriptan is a 5-HT1B/1D agonist. AXS-07 is designed to provide rapid, enhanced and consistent relief of migraine, with reduced symptom recurrence. AXS-07 is covered by more than 80 issued U.S. and international patents which provide protection out to 2036. AXS-07 is not approved by the FDA.
https://pubchem.ncbi.nlm.nih.gov/compound/Rizatriptan
https://pubchem.ncbi.nlm.nih.gov/compound/Meloxicam#section=2D-Structure
Monday, January 3, 2022
Marinus Announces FDA Acceptance for New Drug Application for Ganaxolone in CDKL5 Deficiency Disorder
Marinus Pharmaceuticals, Inc. announced the U.S. FDA acceptance for filing the company’s New Drug Application (NDA) for the use of ganaxolone in the treatment of seizures associated with CDKL5 deficiency disorder (CDD), a rare, genetic epilepsy. The NDA was granted Priority Review designation and the FDA assigned a Prescription Drug User Fee Act (PDUFA) action date of March 20, 2022. Priority Review designation is given to an investigational medicine that, if approved, would be a significant improvement in the safety or effectiveness of the treatment of a serious condition and accelerates the timing of the FDA review of the application compared to a standard review.
“The FDA’s acceptance of our NDA submission is an important step toward potentially bringing the first approved therapy specifically for treatment of seizures associated with CDD—a devastating disorder with high unmet medical need—to families and healthcare providers,” said Scott Braunstein, M.D., Chief Executive Officer of Marinus Pharmaceuticals. “We believe that ganaxolone has the potential to provide meaningful clinical benefit for patients and we look forward to working closely with the FDA during the review process.”
Ganaxolone received orphan drug designation and Rare Pediatric Disease (RPD) designation for CDD in June 2017 and July 2020, respectively. If the NDA is approved, Marinus is eligible to receive a RPD Priority Review Voucher that may be sold or transferred.
In its acceptance letter, the FDA indicated that it is not currently planning to hold an advisory committee meeting to discuss the application.
The acceptance of the NDA for filing enables the company to draw $30 million of additional cash under its May 11, 2021 credit financing agreement with Oaktree Capital Management, L.P., subject to the satisfaction of certain customary conditions described in the credit agreement. If the NDA is approved by December 31, 2022, the company may draw an additional $30 million under the agreement, subject to the satisfaction of certain customary conditions described in the credit agreement.
The NDA is supported by data from the Marigold study, a Phase 3, double-blind placebo-controlled trial in 101 patients. Patients treated with ganaxolone showed a 30.7% median reduction in 28-day major motor seizure frequency, compared to a 6.9% reduction for those receiving placebo, achieving the trial’s primary endpoint (p=0.0036). Patients in the open-label extension study treated with ganaxolone for at least 12 months (n=48) experienced a median 49.6% reduction in major motor seizure frequency. In the Marigold trial, ganaxolone was generally well-tolerated and showed a safety profile consistent with previous clinical trials, with the most frequent adverse event being somnolence.
Marinus has established an Expanded Access Program (EAP) (NCT04678479) for patients in the U.S. who may be eligible to receive access to ganaxolone during the review of the NDA. Additional information about Marinus’ EAP is available here.
https://en.wikipedia.org/wiki/Ganaxolone
Thursday, December 30, 2021
Spero Therapeutics Submits New Drug Application for Tebipenem HBr for the Treatment of Complicated Urinary Tract Infections including Pyelonephritis

Spero Therapeutics, Inc. announced the submission of a new drug application (NDA) to the U.S. Food and Drug Administration (FDA), seeking approval for tebipenem HBr tablets for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, caused by susceptible microorganisms. If approved, tebipenem HBr would be the only oral carbapenem antibiotic available for use in cUTI.
“With the submission of this NDA, we have taken a major step towards potentially providing a substantial number of appropriate cUTI patients with an oral treatment option that could replace historical use of intravenous (IV) therapy,” said Ankit Mahadevia, M.D., Chief Executive Officer of Spero Therapeutics. “If approved, we believe tebipenem HBr could help patients significantly, and the avoidance of IV administration could lead to reduced healthcare resource utilization. We look forward to working with the FDA during the NDA review process as we prepare for tebipenem HBr’s anticipated launch in the second half of 2022.”
The NDA submission includes previously communicated positive data from the Phase 3 ADAPT-PO trial. This data showed that ADAPT-PO met its primary endpoint by demonstrating that oral tebipenem HBr was statistically non-inferior to IV ertapenem in the treatment of patients with cUTI and patients with acute pyelonephritis (AP).
https://pubchem.ncbi.nlm.nih.gov/compound/Tebipenem-pivoxil-hydrobromide
Wednesday, April 29, 2020
FDA Acceptance of ALKS 3831 New Drug Application for Treatment of Schizophrenia and Bipolar Disorder
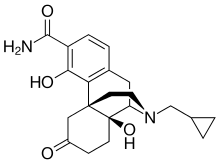
"The acceptance of the NDA for ALKS 3831 marks an important milestone toward our goal of offering a new treatment option to people living with schizophrenia or bipolar I disorder. The ALKS 3831 development program builds on Alkermes' commitment to developing new therapeutic options that seek to address unmet needs of patients in large therapeutic areas," said Craig Hopkinson, M.D., Chief Medical Officer at Alkermes. "We believe ALKS 3831 has the potential to be a meaningful new offering for patients with these serious and complex mental health disorders, and we look forward to engaging with the FDA throughout the NDA review process."
https://en.wikipedia.org/wiki/Olanzapine
https://en.wikipedia.org/wiki/Samidorphan