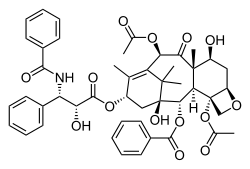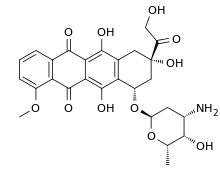
Thursday, July 27, 2017
FDA approves new therapy for initial treatment of soft tissue sarcoma

Thursday, April 24, 2014
Doxorubicin alone or with ifosfamide for treating soft tissue sarcoma? -- ScienceDaily
Tuesday, March 10, 2015
Final Phase 1 data of zoptarelin doxorubicin Phase 1/2 trial published in Clinical Cancer Research
Wednesday, April 8, 2015
PharmaMar to begin PM1183 Phase III trial in combination with doxorubicin in SCLC
Saturday, January 5, 2013
Aeterna Zentaris reaches SPA agreement with FDA for AEZS-108 Phase 3 trial in endometrial cancer
"We are pleased with the agreement with the FDA which provides us with a clearly defined development and regulatory pathway for AEZS-108 in endometrial cancer", stated Juergen Engel , PhD, President and CEO at Aeterna Zentaris. "AEZS-108's innovative targeted approach could offer a new treatment option for women with endometrial cancer and provide the Company with a significant market opportunity."
Tuesday, November 20, 2012
Drug trio of rapamycin, sildenafil and doxorubicin improved effectiveness of cancer treatment, protected heart
"Because sildenafil and rapamycin are clinically approved drugs that both protect heart muscle, we thought that combining these drugs with doxorubicin would be a unique strategy to eliminate the cardiac side effects of doxorubicin while further improving its cancer-killing ability," said Rakesh Kukreja, Ph.D., study co-author and professor of internal medicine and cardiology, Virginia Commonwealth University (VCU) School of Medicine in Richmond.
Thursday, June 22, 2017
Edible ginger-derived nano-lipids could effectively deliver drugs for treating colon cancer
Ref : http://www.nanowerk.com/nanotechnology-news/newsid=44454.php
Friday, November 15, 2013
Combination of heat, doxorubicin drug and nanotech system may improve ovarian cancer treatment
"Ovarian cancer is rarely detected early, and because of that chemotherapy is often needed in addition to surgery," said Oleh Taratula, an assistant professor in the OSU College of Pharmacy. "It's essential for the chemotherapy to be as effective as possible the first time it's used, and we believe this new approach should help with that."
Friday, October 7, 2022
Specific sequence of drugs reduces cost of treating metastatic breast cancer while preserving quality of life
The researchers developed three different computer models to predict how a hypothetical set of 10,000 patients with specific types of metastatic breast cancer would respond to different sequences and types of chemotherapy. For this study, the patient's cancer was either no longer responding to hormone therapies (endocrine resistant) or was a type of the disease called triple-negative breast cancer.
Currently, there are many chemotherapy choices to treat metastatic breast cancer. Oncologists have some preferences of which drugs to use early in treatment, but there is little clear evidence on the best order in which to give the drugs. The researchers consulted oncologists and experts in the field to choose which chemotherapy drugs were preferred choices to include in the study.
Mimicking clinical practice, and based upon existing data, the researchers then assumed that if a person started treatment with one drug, they would change to a second-choice treatment after their cancer stopped responding to the first drug, or if the side effects weren't tolerable. The purpose of the study was to test whether putting the drugs in one sequence compared to another could keep the patient on treatment for similar times while decreasing their side effect and/or cost burden.
"The cost of cancer drugs in the U.S. has rapidly increased, even for generics. As a society, we urgently need more strategies to reduce cancer drug costs without compromising outcomes, and our analysis provides quantifiable evidence to help providers choose lower priced, but equally effective sequences of drugs," said Stephanie B. Wheeler, PhD, MPH, professor of health policy & management at UNC Gillings and associate director of community outreach and engagement at UNC Lineberger and corresponding author of the article. "More spending on cancer care does not necessarily confer greater health benefits."
The costs calculated in this study were inclusive of medical and nonmedical costs borne by patients, including lost productivity. In this simulation, after two years, nearly all women would have completed the first three sets of treatment, but the cancer would cause the death of about one-third of the women. Productivity days lost due to sickness were similar across chemotherapy sequences, so most of the cost difference was due to drug savings. In the simulation, patients were placed in three groups, depending on what treatments they had already received for earlier episodes of breast cancer.
Outcomes in the three groups were:
- For people who had not previously received the common chemotherapy drug categories, including a taxane (e.g., paclitaxel) or an anthracycline (e.g., capecitabine), treatment with paclitaxel then capecitabine followed by doxorubicin corresponded to the highest expected gains in quality of life and lowest costs.
- For people who had previously received a taxane and an anthracycline drug, treatment with carboplatin, followed by capecitabine, followed by eribulin, corresponded to the highest expected gains in quality of life and lowest costs.
- For people who had previously received a taxane but not an anthracycline, treatment sequences beginning with capecitabine or doxorubicin, followed by eribulin, were most cost-effective.
"The drugs we studied are already recommended and reimbursed for the treatment of metastatic breast cancer, but the optimal sequencing of them has been unclear, which has led to considerable variation in physician preference and practice. Our study suggests that treatment sequencing approaches that minimize costs early may improve the value of care," Wheeler said. "The implications of this study are fairly straightforward for medical oncologists and those developing value-based clinical pathways to implement in practice now."
UNC Lineberger's Katherine E. Reeder-Hayes, MD, MBA, MSc, section chief of breast oncology and associate professor of medicine at UNC School of Medicine and one of the study's authors, said the treatment choices for metastatic breast cancer are constantly changing, and new options for targeted therapy have emerged even since this study was conducted. "Many oncologists and patients find that there aren't any more targeted therapies that fit the cancer's molecular profiles, so they are left with the choice of a number of chemotherapy drugs that may feel pretty similar or have an unclear balance of pros and cons.
"In that scenario, I hope our study will help expand the framework that we use to make these decisions from one where we just think about the biologic action of the drug to one where we also consider the bigger picture of what the treatment experience is like for the patient, including their financial burden, investment of time, and side effects," Reeder-Hayes added. "The most potent drug isn't always the next best choice depending on what the patient values and wants to accomplish with their treatment."
Looking ahead, the researchers have developed a financial navigation program to further support patients in managing the out-of-pocket costs of their cancer care. This program has been effective and well received by patients, caregivers and providers. The team is currently scaling up the intervention in nine rural and non-rural oncology practices across North Carolina to understand how well it works in different care settings. Cancer patients who need financial support managing the cost of their cancer care are being recruited for this undertaking.
Tuesday, February 4, 2020
Epizyme Announces FDA Filing Acceptance of New Drug Application and Priority Review for Tazemetostat for the Treatment of Epithelioid Sarcoma
“We are thrilled with FDA’s acceptance of this first tazemetostat NDA submission for priority review, and to be an important step closer to achieving our mission of rewriting treatment for patients with cancer and other serious diseases,” said Robert Bazemore, president and chief executive officer of Epizyme. “This is a significant achievement in the development of this potentially first-in-class EZH2 inhibitor, and we look forward to working with FDA during the review. If approved, we believe tazemetostat could become an important new option in the treating physicians’ arsenal. We would like to extend our sincerest gratitude to those patients, families and medical teams who have participated in our clinical studies and helped bring tazemetostat to this stage.”
Thursday, April 23, 2020
FDA Approves Tazverik (tazemetostat) for the Treatment of Patients with Epithelioid Sarcoma
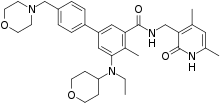
“For people with epithelioid sarcoma, an aggressive life threatening cancer that affects young adults, having new treatment options can offer much needed hope,” added Denise Reinke, MS, NP, MBA, president and chief executive officer of the Sarcoma Alliance for Research through Collaboration (SARC) and co-founder of the Sarcoma Coalition.
Saturday, September 12, 2009
Friday, December 5, 2014
Chemotherapy drug combined with cancer-killing virus may treat recurrent ovarian cancer
Thursday, November 5, 2009
Lovastatin-synthesizing enzyme successfully reconstituted...

Dield-Alder catalysed cyclisation : In vitro formation of a triketide lactone using a genetically-modified protein derived from 6-deoxyerythronolide B synthase has been demonstrated. The stereochemistry of the molecule supports the intriguing idea that an enzyme-catalyzed Diels-Alder reaction may occur during assembly of the polyketide chain. It thus appears that biological Diels-Alder reactions may be triggered by generation of reactive triene systems on an enzyme surface.
Biosynthesis using broadly specific acyltransferase : It has been found that a dedicated acyltransferase, LovD, is encoded in the lovastatin biosynthetic pathway. LovD has a broad substrate specificity towards the acyl carrier, the acyl substrate and the decalin acyl acceptor. It efficiently catalyzes the acyl transfer from coenzyme A thoesters or N-acetylcysteamine (SNAC) thioesters to monacolin J. The biosynthesis of lovastatin is coordinated by two iterative type I polyketide syntheses and numerous accessory enzymes. Nonketide, the intermediate biosynthetic precursor of lovastatin, is assembled by the upstream megasynthase LovB (also known as lovastatin nonaketide synthase), enoylreductase LovC, and CYP450 oxygenases.
Recently more interesting out come from a group of UCLA researchers is that, for the first time thy have successfully reconstituted in the laboratory the enzyme responsible for producing the blockbuster cholesterol-lowering drug lovastatin. As per the claim by the researchers, the lovastatin-synthesizing enzyme is one of the most interesting but least understood of the polyketide synthases, which are found in filamentous fungi and which play a crucial role in the synthesis of "small molecule natural products" — pharmacologically or biologically potent compounds produced by living organisms, many of which are the active ingredients in pharmaceuticals.
This finding is of great significance because commonly used antibiotics, such as tetracycline, are produced by polyketide synthases. Polyketides represent a class of 7,000 known structures, of which more than 20 are commercial drugs, including the immunosuppressant rapamycin, the antibiotic erythromycin and the anticancer drug doxorubicin. In their study studied the enzyme that makes a small-molecule precursor to lovastatin. The real difference about this enzyme, is its extraoridnarily large size in comparison to all other enzymes so for studied. As per the claim by the lead researcher Dr. Yi Tang, "It's one of the largest enzymes ever to be reconstituted in a test tube. It is 10 times the size of most enzymes people study & the enzyme has seven active sites and catalyzes more than 40 different reactions that eventually result in an important precursor to lovastatin. Hope with this remarkable achievement, one can prepare many natural products in the lab in the days to come.
Ref : http://www.newsroom.ucla.edu/portal/ucla/ucla-engineering-researchers-have-111812.aspx
Monday, January 8, 2018
New cancer drug begins clinical trial in human patients with rare brain tumor
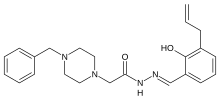
"Most cancers have elevated levels of procaspase-3," Hergenrother said. "When it is turned on, procaspase-3 kills cells."
"PAC-1 restores the activation of procaspase-3 and, because this enzyme is elevated in cancer cells, targets cancer cells over noncancerous cells," he said.
"This requires that the rodents be immunocompromised to mitigate rejection of human cells," he said. "As such, most rodent tumor models do not faithfully recapitulate the tumor microenvironment - in particular, the body's immune surveillance of the tumor.
"I look at pets with spontaneous tumors as being complementary to rodent models and recognize that not all discoveries in pet dogs will necessarily translate similarly to people," Fan said.
"Glioblastoma multiforme has this feature of spreading silently along the blood vessels inside the brain," he said. "That's a reason why most patients will unfortunately have disease coming back later on after surgery and radiation."
"All three dogs had, at the very least, what we call a partial response, which means more than a 30 percent reduction in the tumor," he said. "And one of the dogs had a complete response, as identified with serial MRI scans, with a 100 percent reduction in the tumor mass 84 days after combination therapy."
Wednesday, January 8, 2014
2 Pre-Surgery Drug Treatments Show Promise Against Aggressive Breast Cancer - Drugs.com MedNews
Thursday, April 20, 2017
Everolimus combined with standard R-CHOP therapy shows promise in treating DLBCL patients

"There is an unmet need to develop new therapies based on R-CHOP to try to increase the cure rate for diffuse large B-cell lymphoma," says Patrick Johnston, M.D., Ph.D., a hematologist at Mayo Clinic and lead author. "This pilot study suggests that adding mTOR inhibitors to standard therapy could improve outcomes, though it needs to be validated in a larger clinical trial."
"This study is the first to integrate a P13K-mTOR agent with standard RCHOP," says Dr. Johnston. "The encouraging outcome results and toxicity profile of this new regimen, along with the worldwide availability of everolimus, make it potentially applicable to the large population of DLBCL patients."
Wednesday, August 17, 2016
New drug combination before surgery may improve outcomes in breast cancer patients