Tuesday, March 23, 2021
Chinese scientists ask for patent on US drug to fight virus

Saturday, January 23, 2021
Researchers identify three drugs as possible therapeutics for COVID-19
In continuation of my update on Amodiaquine and nebivolol
Based on virtual and in vitro antiviral screening that began in the earlier months of the COVID-19 pandemic, the researchers led at UTHSC by Colleen Jonsson, PhD, identified zuclopenthixol, nebivolol, and amodiaquine as promising therapeutics for the virus in its early stages.
Dr. Jonsson is a professor and the Endowed Van Vleet Chair of Excellence in Virology in the College of Medicine at UTHSC. She also directs the UTHSC Regional Biocontainment Laboratory (RBL), where this research was conducted. The university's RBL is one of roughly a dozen federally funded labs authorized to safely study contagious pathogens.
In a paper published in ACS Pharmacology & Translational Science, the researchers propose the drugs as possible candidates for testing in future clinical trials to improve immune response to the virus. Amodiaquine is an older antimalarial, zuclopenthixol is an antipsychotic, and nebivolol is a blood pressure medication.
"Particularly in the context of this pandemic, there is a stringent need for high-quality studies that can provide critical knowledge concerning the COVID-19 disease and reliable treatment proposals," the paper states. "With these caveats in mind, we conceived a computational workflow that included independent in vitro validation, followed by assessing emerging candidates in the context of available clinical pharmacology data with the aim of proposing suitable candidates for clinical studies for early stage (incubation and symptomatic phases) patients infected by SARS-CoV-2."
"Given the need for improved efficacy and safety, we propose zuclopenthixol, nebivolol, and amodiaquine as potential candidates for clinical trials against the early phase of the SARS-CoV-2 infection," the researchers wrote.
Comparing the drugs to hydroxychloroquine, the anti-malarial drug most-frequently studied in clinical trials for use as a COVID-19 therapeutic, the researchers examined 4,000 approved drugs and found these three to act similarly to the hydroxychloroquine, and in some cases, more safely. The research indicates they may also improve efficacy when combined in lower doses with remdesivir, an anti-viral given an emergency use authorization by the United States Food and Drug Administration as a therapeutic for COVID-19.
"Think of it as a whack-a-mole game," said Tudor Oprea, MD, PhD, professor of Medicine and Pharmaceutical Sciences, chief of the UNM Division of Translational Informatics, and corresponding author on the paper. "Instead of having one hammer, you have two hammers, which is more effective. We're trying to give the scientific community two hammers, instead of one."
Dr. Jonsson added, "This is a very exciting discovery and we are following up on the potential use of zuclopenthixol, nebivolol, and amodiaquine in additional research studies."
Monday, January 11, 2021
FDA Issues EUA to Baricitinib Plus Remdesivir for COVID-19
In continuation of my update on baricitinib and remdesivir
Emergency use authorization was issued for baricitinib in combination with remdesivir for hospitalized patients with COVID-19, the U.S. Food and Drug Administration announced Thursday.
The EUA for the combination treatment applies to hospitalized patients ages 2 years and older with suspected or laboratory-confirmed COVID-19 who require supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation. The janus kinase inhibitor baricitinib is currently FDA-approved for treating moderately to severely active rheumatoid arthritis.
Based on the agency's review of the evidence, the FDA "determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population. And, when used under the conditions described in the EUA to treat COVID-19, the known and potential benefits of baricitinib outweigh the known and potential risks for the drug."
The FDA granted the EUA based on data from the ACTT-2 trial, a randomized, double-blind, placebo-controlled clinical trial conducted by the National Institute of Allergy and Infectious Diseases. The trial included 1,033 patients -- 515 randomly assigned to baricitinib plus remdesivir and 518 randomly assigned to placebo plus remdesivir. Patients were followed for 29 days. Median time to recovery from COVID-19 was seven and eight days for patients receiving baricitinib plus remdesivir and those receiving placebo plus remdesivir, respectively. Patients receiving baricitinib plus remdesivir had significantly lower odds of progressing to death or being ventilated at 29 days and significantly higher odds of clinical improvement at 15 days compared with patients receiving placebo plus remdesivir.
Baricitinib is not authorized or approved as a stand-alone treatment for COVID-19, the FDA notes. Its safety and effectiveness for use in the treatment of COVID-19 continue to be evaluated.
https://en.wikipedia.org/wiki/Baricitinib
https://en.wikipedia.org/wiki/Remdesivir
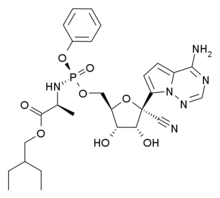
Monday, July 6, 2020
Experimental Antiviral Drug to Be Tested Against New Coronavirus

Friday, February 14, 2020
Experimental antiviral prevents MERS-CoV in rhesus macaques

https://en.wikipedia.org/wiki/Remdesivir
Tuesday, January 28, 2020
AbbVie's HIV Drug Aluvia (Lopinavir/ritonavir) Seen as Potential Treatment for Coronavirus






