Good old aspirin is just as effective as newer, expensive drugs at preventing blood clots after hip or knee replacement, a new clinical trial suggests.
Researchers said the findings could change some doctors' prescribing habits.
After knee or hip replacement surgery, there's a risk of blood clots in the legs or lungs. So it's routine for patients to take clot-preventing drugs for some time afterward.
Right now, some doctors choose powerful anti-clotting drugs like dabigatran (Pradaxa) and rivaroxaban(Xarelto), said Dr. David Anderson, the lead researcher on the new trial.
But it hasn't been clear whether those expensive prescription drugs are any better than cheap, readily available aspirin, explained Anderson, of Dalhousie University, in Halifax, Canada.
Based on the new findings, they're not.
Few patients in the study developed a blood clot after surgery, and those on aspirin fared just as well as those on rivaroxaban.
The caveat, Anderson said, was that all study patients received rivaroxaban for the first five days after surgery. After that, they either continued on the drug or switched to aspirin for another nine to 30 days.
"From this study, we have no evidence to support starting aspirin on day one," Anderson said.
But after day five, he added, "it's very reasonable to consider switching to aspirin."
Over the past decade, surgeons have already been turning away from powerful anticoagulants toward aspirin and non-drug options for thwarting clots, said Dr. Alejandro Gonzalez Della Valle.
Gonzalez Della Valle specializes in hip and knee surgery at the Hospital for Special Surgery in New York City.
These days, he said, patients have a generally low risk of blood clots after hip or knee replacement for a number of reasons. Those include shorter surgical times, and the use of regional anesthesia instead of general.
Clots can also be prevented by improving blood flow in patients' legs right after surgery. So getting patients on their feet and moving early on is key, Gonzalez Della Valle explained. Similarly, pneumatic compression devices can be used to encourage blood flow in the lower limbs while patients are in their hospital beds.
Dr. Kevin Bozic, a spokesperson for the American Academy of Orthopaedic Surgeons (AAOS), said that the AAOS guidelines already state that no one drug is better than another for preventing clots.
"This study reinforces that," Bozic said.
He agreed that most surgeons have been turning to aspirin in the past 10 years because recovery times are shorter and people leave the hospital much sooner. Most people can have just aspirin, but some at high risk of blood clots -- those with a history of clots, people who are very obese -- might need an anticoagulant, Bozic added.
"The strategy for preventing clots should include medication and early mobilization," he stressed.
Ref:http://www.nejm.org/doi/full/10.1056/NEJMoa1712746
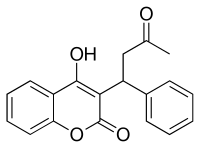
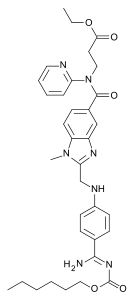 Dabigatran
Dabigatran  Rivaroxaban (BAY 59-7939)
Rivaroxaban (BAY 59-7939)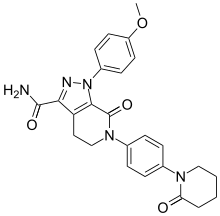 Apixaban
Apixaban  Edoxaban
Edoxaban