Thursday, August 29, 2024
Drug Used to Treat Rheumatoid Arthritis May Also Help Prevent It
Wednesday, July 14, 2021
Drug could be promising new option against eczema
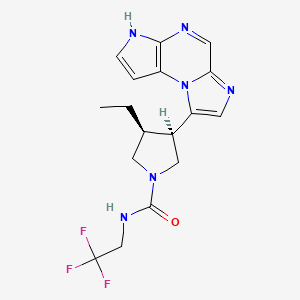
In continuation of my update on Upadacitinib
A pill called upadacitinib, already approved for treating rheumatoid arthritis, might also ease another common immunological condition—eczema.
In two phase 3 clinical trials, patients with moderate to severe eczema showed rapid and significant improvements after taking the drug, said researchers at Mount Sinai in New York City.
The clinical trials were funded by the dug's maker, AbbVie Inc., and included nearly 1,700 patients with the inflammatory skin condition.
"The results of these trials ... were so incredible that by week 16, most patients with moderate to severe atopic dermatitis [eczema] either had a 90% disease clearance, or even 100% disease clearance," study first author Dr. Emma Guttman-Yassky said in a Mount Sinai news release. She's professor and chair of the department of dermatology at Mount Sinai's Icahn School of Medicine, in New York City.
"We achieved extremely high clearance rates that are bringing us closer to the amazing clearance rates that we see in psoriasis," Guttman-Yassky noted.
According to the National Eczema Association, "people with eczema tend to have an over-reactive immune system that when triggered by a substance outside or inside the body, responds by producing inflammation. It is this inflammation that causes the red, itchy and painful skin symptoms common to most types of eczema."
Eczema affects more that 31 million American adults and between 10 to 20% of children, the study authors noted.
The two new clinical trials involved a total of almost 1,700 patients and took place between 2018 and 2020.
Besides the rapid disease clearance noted in patients, "the itch improvements already started to be significant within days from the beginning of the trials, and the maximum clinical efficacy was obtained early, at week 4, and maintained to week 16," Guttman-Yassky said.
The drug was well tolerated by patients who received the two highest doses of the drug—15 milligrams and 30 milligrams—and no significant safety risks were seen, she added.
Upadacitinib is already approved and marketed for use against rheumatoid arthritis under the brand name Rinvoq. It works by blocking what are known as multiple cytokine-signaling pathways—parts of the immune system that can malfunction and cause eczema.
According to Guttman-Yassky, other eczema therapies exist, but most come with certain drawbacks.
While injectable biologic drugs are highly successful in treating eczema patients who don't respond to or can't use topical creams, their use cannot be stopped and restarted at will, because the potential creation of anti-drug antibodies will shorten the half-life of the drugs, she explained.
However, "patients were able to start and restart [upadacitinib] at any time, allowing for flexibility, which cannot be achieved with biologics," Guttman-Yassky, said. "And, biologics, which are injectable agents that target specific lymphocytes that are 'misbehaving' or are up-regulated in atopic dermatitis, do not suppress the entire immune system as other immunosuppressants tend to do."
Dr. Michele Green is a dermatologist at Lenox Hill Hospital in New York City who wasn't involved in the new study.
She called the findings "important."
Upadacitinib is the first drug in its class "to be effectively used for patients with significant improvement of pruritus [itch] within several days of treatment and clearance of their disease within several weeks," Green noted.
"It is also significant since adolescents were included in this study and I believe an oral treatment is much more appealing to treating adolescents than current injectable biologics," she added.
https://pubchem.ncbi.nlm.nih.gov/compound/Upadacitinib#section=2D-Structure
Monday, January 11, 2021
FDA Issues EUA to Baricitinib Plus Remdesivir for COVID-19
In continuation of my update on baricitinib and remdesivir
Emergency use authorization was issued for baricitinib in combination with remdesivir for hospitalized patients with COVID-19, the U.S. Food and Drug Administration announced Thursday.
The EUA for the combination treatment applies to hospitalized patients ages 2 years and older with suspected or laboratory-confirmed COVID-19 who require supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation. The janus kinase inhibitor baricitinib is currently FDA-approved for treating moderately to severely active rheumatoid arthritis.
Based on the agency's review of the evidence, the FDA "determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population. And, when used under the conditions described in the EUA to treat COVID-19, the known and potential benefits of baricitinib outweigh the known and potential risks for the drug."
The FDA granted the EUA based on data from the ACTT-2 trial, a randomized, double-blind, placebo-controlled clinical trial conducted by the National Institute of Allergy and Infectious Diseases. The trial included 1,033 patients -- 515 randomly assigned to baricitinib plus remdesivir and 518 randomly assigned to placebo plus remdesivir. Patients were followed for 29 days. Median time to recovery from COVID-19 was seven and eight days for patients receiving baricitinib plus remdesivir and those receiving placebo plus remdesivir, respectively. Patients receiving baricitinib plus remdesivir had significantly lower odds of progressing to death or being ventilated at 29 days and significantly higher odds of clinical improvement at 15 days compared with patients receiving placebo plus remdesivir.
Baricitinib is not authorized or approved as a stand-alone treatment for COVID-19, the FDA notes. Its safety and effectiveness for use in the treatment of COVID-19 continue to be evaluated.
https://en.wikipedia.org/wiki/Baricitinib
https://en.wikipedia.org/wiki/Remdesivir
Thursday, February 20, 2020
FDA Approves RediTrex (methotrexate) for Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, and Psoriasis

Thursday, January 23, 2020
FDA Approves Rinvoq (upadacitinib), an Oral JAK Inhibitor for the Treatment of Moderate to Severe Rheumatoid Arthritis

"Despite the availability of multiple treatment options with varying mechanisms of action, many patients still do not achieve clinical remission or low disease activity—the primary treatment goals for rheumatoid arthritis," said Roy M. Fleischmann, M.D., primary investigator for SELECT-COMPARE and clinical professor at the University of Texas Southwestern Medical Center at Dallas. "With this FDA approval, Rinvoq has the potential to help additional people living with RA achieve remission who have not yet reached this goal."
- In SELECT-EARLY, 52 percent of MTX-naïve patients treated with Rinvoq 15 mg achieved ACR50 vs 28 percent treated with MTX at week 121
- In SELECT-MONOTHERAPY, 68 percent of MTX-IR patients treated with Rinvoq 15 mg achieved ACR20 vs 41 percent treated with continued MTX at week 141
- In SELECT-COMPARE, 71 percent of MTX-IR patients treated with Rinvoq 15 mg plus MTX achieved ACR20 vs 36 percent treated with placebo plus MTX at week 121
- In SELECT-NEXT, 64 percent of csDMARD-IR patients treated with Rinvoq 15 mg plus csDMARDs achieved ACR20 vs 36 percent treated with placebo plus csDMARDs at week 121
- In SELECT-BEYOND, 65 percent of biologic-IR patients treated with Rinvoq 15 mg plus csDMARDs achieved ACR20 vs 28 percent treated with placebo plus csDMARDs at week 121
"The discovery and development of Rinvoq is indicative of AbbVie's long-standing commitment to advancing the science for people living with immune-mediated conditions," said Michael Severino, M.D., vice chairman and president, AbbVie. "Today's FDA approval marks an important milestone in our pursuit to deliver innovative medicines that advance care for people living with rheumatoid arthritis."
Safety
Ease of Use and Access
"Rheumatoid arthritis can have a debilitating impact on the lives of those with the chronic disease, including making it difficult to perform everyday tasks," said Cindy McDaniel, senior vice president, consumer health, Arthritis Foundation. "The Arthritis Foundation is committed to recognizing innovation that can help patients living with rheumatoid arthritis and we are proud to recognize AbbVie with our Ease of Use Commendation for the packaging design of Rinvoq."
Thursday, August 15, 2019
FDA Approves Boxed Warning About Increased Risk of Blood Clots and Death with Higher Dose of Tofacitinib (Xeljanz, Xeljanz XR)
- Sudden shortness of breath
- Chest pain that worsens with breathing
- Swelling of a leg or arm
- Leg pain or tenderness, or red or discolored skin in the painful or swollen leg or arm
- 19 cases of blood clots in the lung out of 3,884 patient-years of follow-up in patients who received tofacitinib 10 mg twice daily, compared to 3 cases out of 3,982 patient-years in patients who received TNF blockers
- 45 cases of death from all causes out of 3,884 patient-years of follow-up in patients who received tofacitinib 10 mg twice daily, compared to 25 cases out of 3,982 patient-years in patients who received TNF blockers
Friday, March 22, 2019
AbbVie Announces New Drug Application Accepted for Priority Review by FDA for Upadacitinib for Treatment of Moderate to Severe Rheumatoid Arthritis

About the SELECT Study Program
About Upadacitinib
Tuesday, February 26, 2019
Rituximab (Rituxan) May Delay MS Disability
"This is a potentially valuable treatment, but there are still a lot of questions. Other studies are underway looking at the value of rituximab," LaRocca said.
Saturday, February 23, 2019
Psoriasis Meds Might Help Fight Heart Trouble, Too
"Classically a heart attack is caused by one of five risk factors: diabetes, hypertension, high cholesterol, family history or smoking," explained study lead researcher Dr. Nehal Mehta.
"Our study presents evidence that there is a sixth factor, inflammation," she said.
"The future of cardiovascular prevention may require a cholesterol reduction medication and an anti-inflammatory medication," said Dr. Guy Mintz, who directs heart health at Northwell Health's Sandra Atlas Bass Heart Hospital in Manhasset, N.Y.
"These are exciting times in the area of cardiovascular prevention," said Mintz, who wasn't involved with the study.
"This appears to be an anti-inflammatory effect," Mehta explained in an NHLBI news release. "In the absence of improvement in other cardiovascular risk factors, and without adding new cholesterol medications, patients' soft plaque still improved."
"The best statin in the world can only lower cardiovascular events by approximately 40 percent," Mintz pointed out. "So the question arises, what causes the other 60 percent of cardiovascular events?"
Wednesday, September 5, 2018
FDA Approves Olumiant (baricitinib) 2 mg Tablets for the Treatment of Adults with Moderately-to-Severely Active Rheumatoid Arthritis

"We are pleased to provide RA patients in the U.S. an effective treatment option with Olumiant, as people with RA who have had an inadequate response to TNF inhibitors are generally considered to be some of the most difficult to treat RA patients," said Christi Shaw, president, Lilly Bio-Medicines.
"Despite the advancements we've seen in the RA treatment landscape over the past several decades, many patients are still failing to achieve their disease management goals," said Seth Ginsberg, co-founder and president of CreakyJoints and the Global Healthy Living Foundation. "As it's important for RA patients to have multiple treatment options available to best suit their disease characteristics and experiences, the approval of Olumiant is very encouraging for our community."
"In my clinical practice, I continue to see patients who experience debilitating symptoms and who are waiting for a medicine that may be right for them," said Elizabeth L. Perkins, M.D., Rheumatology Care Center, Birmingham, Alabama. "Olumiant is an important option for rheumatologists to help address these patients' unmet needs."
"RA patients continue to experience unique challenges accessing the treatments prescribed by their healthcare providers. Therefore, we are determined to continue our work with stakeholders to demonstrate value across the healthcare system so providers have greater choice in prescribing treatments to fit individual patient needs," said Shaw.
Thursday, August 23, 2018
FDA Advisory Committee Recommends the Approval of Baricitinib 2mg, but not 4mg, for the Treatment of Moderately-to-Severely Active Rheumatoid Arthritis
"We are confident that baricitinib, if approved, can help people in the U.S. manage the challenges of living with RA," said Christi Shaw, president of Lilly Bio-Medicines. "While we are disappointed with the Advisory Committee's assessment of the data for the 4-mg dose, we are confident in the positive benefit-risk profile of both the 2-mg and the 4-mg doses. We look forward to continuing our work with the FDA on our New Drug Application (NDA) and are hopeful that baricitinib will receive approval in the coming months."
"Despite advances in the management of RA over the last 20 years, which include early treatment, optimized use of traditional therapies for rheumatic disease and the advent of newer medications such as biologics, many patients are still struggling to meet treatment targets, and live with debilitating pain, fatigue and other symptoms of RA," said Peter Taylor, MA, PhD, professor, University of Oxford, an expert who attended the Advisory Committee Meeting. "Baricitinib could be a promising option for RA patients in the U.S. who are not achieving adequate disease control with currently available treatments."