Monday, August 5, 2024
Liquidia Corporation Provides Update on New Drug Application for Yutrepia (treprostinil) inhalation powder
Thursday, December 23, 2021
FDA Grants Tentative Approval for Liquidia’s Yutrepia (treprostinil) Inhalation Powder
In continuation of my update on treprostinil
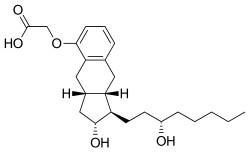
Liquidia Corporation announced that, the U.S. Food and Drug Administration (FDA) has granted tentative approval for Yutrepia™ (treprostinil) inhalation powder, previously referred to as LIQ861. Yutrepia is indicated for the treatment of pulmonary arterial hypertension (PAH) to improve exercise ability in adult patients with New York Heart Association (NYHA) Functional Class II-III symptoms. Tentative approval indicates that Yutrepia has met all regulatory standards for quality, safety and efficacy required for approval in the United States.
Dr. Tushar Shah, Chief Medical Officer of Liquidia, said: “We would like to take the opportunity to thank the patients and investigators who participated in the clinical development of Yutrepia. The tentative approval for Yutrepia is another step toward providing an important option for patients with PAH in the U.S. We believe Yutrepia can improve the limitations of current nebulized therapies by allowing the administration of an expanded dose range of inhaled treprostinil using a proven, convenient, palm-sized device.”
The addressable market for inhaled treprostinil is significant and expected to grow. In 2020, United Therapeutics reported that its nebulized formulation of treprostinil indicated for PAH achieved sales of more than $480 million. The attributes of Yutrepia including ease-of-use, convenience, direct lung delivery, and higher dosage range may not only make Yutrepia a preference to nebulized therapy, but also an alternative to oral treatments, and possibly a treatment option to delay the use of parenteral therapies in PAH. There may also be future expansion opportunities for inhaled treprostinil into additional indications.
Damian deGoa, Chief Executive Officer of Liquidia added: “This is a significant milestone for Liquidia. We are really proud of our team. Not only does the tentative approval establish the safety and efficacy of Yutrepia for PAH patients but, in the process, we have validated our proprietary PRINT® technology to engineer discrete drug particles with uniform composition, size, and shape. There is more work to be done. We will now focus our efforts on pre-commercial launch activities and the growing market opportunity for Yutrepia in PAH and potential new indications.”
Due to a regulatory stay pursuant to the Drug Price Competition and Patent Term Restoration Act (Hatch-Waxman Act), Yutrepia cannot yet be marketed in the United States. In June 2020, United Therapeutics filed a lawsuit against Liquidia for alleged infringement of three patents related to Tyvaso®. As a result, the FDA cannot give final approval of Yutrepia until the expiration of the regulatory stay on October 27, 2022, or earlier resolution or settlement of the ongoing litigation.
About Yutrepia™(treprostinil) inhalation powder
Yutrepia is an investigational, inhaled dry powder formulation of treprostinil delivered through a proven, convenient, palm-sized device. On November 5, 2021, the FDA issued a tentative approval for Yutrepia, which is indicated for the treatment of pulmonary arterial hypertension (PAH) to improve exercise ability in adult patients with New York Heart Association (NYHA) Functional Class II-III symptoms. Yutrepia was designed using Liquidia’s PRINT® technology, which enables the development of drug particles that are precise and uniform in size, shape, and composition, and that are engineered for optimal deposition in the lung following oral inhalation. Liquidia has completed INSPIRE, or Investigation of the Safety and Pharmacology of Dry Powder Inhalation of Treprostinil, an open-label, multi-center phase 3 clinical study of Yutrepia in patients diagnosed with PAH who are naïve to inhaled treprostinil or who are transitioning from Tyvaso (nebulized treprostinil). Yutrepia was previously referred to as LIQ861 in investigational studies.
Saturday, October 20, 2018
United Therapeutics Announces FDA Approval of the Implantable System for Remodulin

"We are extremely excited to offer this new option to patients suffering from PAH," said Martine Rothblatt, Ph.D., Chairman and Chief Executive Officer of United Therapeutics. "During the course of the DelIVery study, we received considerable physician and patient interest in the ISR. We are grateful to our collaborators at Medtronic for reaching this milestone and look forward to continuing our collaboration."
"External infusion pumps have been used to deliver prostacyclins for PAH, but managing the therapy places a significant burden on patients, interferes with their daily activities, and runs a high risk of infections," said David Steinhaus, M.D., general manager of the Heart Failure business, part of the Cardiac and Vascular Group at Medtronic. "This fully implantable drug delivery system was designed to address these serious patient care concerns."
Sunday, December 20, 2009
Treprostinil as IV infusion reduces breathlessness in PAH patients...
 The treatment, continuous intravenous (IV) treprostinil, (see structure, the drug has already been approved by the U.S. Food and Drug Administration for the treatment of pulmonary arterial hypertension PAH) based on its similarity to an approved treatment delivered subcutaneously (directly into the skin). Practicing physicians, had hesitated to endorse the treatment because it did not have its own placebo-controlled study. But now the researchers from University of Rochester Medical Center, have come up with interesting results.
The treatment, continuous intravenous (IV) treprostinil, (see structure, the drug has already been approved by the U.S. Food and Drug Administration for the treatment of pulmonary arterial hypertension PAH) based on its similarity to an approved treatment delivered subcutaneously (directly into the skin). Practicing physicians, had hesitated to endorse the treatment because it did not have its own placebo-controlled study. But now the researchers from University of Rochester Medical Center, have come up with interesting results.The more interesting part of their research is that PAH patients had higher than normal blood levels of factors known to play central roles in the clogging of arteries as part of major diseases like atherosclerosis and hypertension, including angiopoietin-2 (Ang-2) and platelet derived growth factor. Treatment with treprostinil was associated with lowers levels of Ang-2.
Though the mode of action has to be established (relaxing muscles surrounding blood vessels for easier blood flow and turning off sticky ingredients that cause blood clots e.g. platelets) , its is a good achievement. Treprostinil-treated patients feel like they are breathing easier because their lung arteries, not the lungs themselves, are working more efficiently. Better understanding of the mechanisms involved may lead to refinements in drug design; for example, blocking the effects of Ang-2 to treat the disease (may be easier on patients than a continuous IV infusion). Though further studies are essential, its a good achievement and hope in the days to come people with PHT will definitely breathe a sigh of relief instead of breathlessnessssss.....
Source : http://www.urmc.rochester.edu/news/story/index.cfm?id=2711

