In continuation of my update Lonsurf
The U.S. Food and Drug Administration today approved Lonsurf (a pill that combines two drugs, trifluridine and tipiracil) for patients with an advanced form of colorectal cancer who are no longer responding to other therapies.
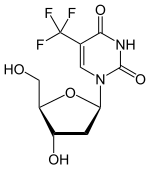 (Trifluridine)
(Trifluridine) 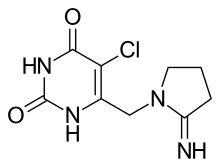 (Tipiracil)
(Tipiracil)
"The past decade has brought a new understanding around colorectal cancer, in how we can both detect and treat this often devastating disease," said Richard Pazdur, M.D., director of the Office of Hematology and Oncology Products in the FDA's Center for Drug Evaluation and Research. "But there are many patients who still need additional options, and today's approval is a testament to the FDA's commitment to work with companies to develop new drugs in disease areas where unmet needs remain."
Colorectal cancer is the third most common non-skin cancer in men and women in the U.S., according to the National Cancer Institute. While still the second leading cause of cancer-related death in the U.S., over the past 10 years the number of colorectal cancer cases and related deaths have decreased, due in part to screenings, such as colonoscopies.










