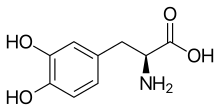
In continuation of my update on Empagliflozin
For the first time, research shows that a type 2 diabetes drug significantly reduces hospitalizations and death from heart failure.
The findings, from a large clinical trial known as EMPA-REG OUTCOME, were presented by Yale professor of medicine and clinical chief of endocrinology, Dr. Silvio E. Inzucchi, at the 2015 American Heart Association (AHA) Scientific Session in Orlando, Florida on Nov. 9.
Many individuals with type 2 diabetes also have heart failure, a condition in which the heart fails to pump blood effectively. Treatment for heart failure is limited and prior efforts to treat patients with type 2 diabetes drugs showed no benefit for heart failure. But a new class of type 2 diabetes drugs (SGLT2 inhibitors) that reduce blood sugar by increasing its excretion in the urine had not been studied.
In the EMPA-REG trial, patients with type 2 diabetes and risk factors for heart disease were randomized to receive once-daily doses of either the glucose-lowering drug empagliflozin (10 mg or 25 mg doses), or a placebo. The drug or placebo was given in addition to standard care.
At the end of the trial period, investigators found that patients treated with the drug experienced reductions in blood sugar and blood pressure, as well as weight loss, compared to those on placebo. They also found major significant reductions in hospitalizations for heart failure (35%); the combined result for heart failure hospitalization or dying from heart disease (34%); and the combined result for being hospitalized or dying from heart failure (39%).