
The Montmorency cherry is a variety of sour cherry (Prunus cerasus) grown in the United States, Canadaand France, particularly in Michigan and in Door County, Wisconsin. Montmorency cherries are part of the lighter-red Amarelle cultivar of sour cherries, rather than the darker-red Morello cultivar. Michigan produces over 90,000 tons of Montmorency cherries each year.
Drinking tart Montmorency cherry juice significantly reduces high blood pressure at a level comparable to that achieved by medication, according to new research from Northumbria University, Newcastle.
The findings, which are published in The American Journal of Clinical Nutrition today (Wednesday 4 May), found that men with early signs of hypertension - more commonly known as high blood pressure - saw a 7% reduction in blood pressure after drinking Montmorency cherry concentrate when compared to drinking a fruit-flavoured cordial.
This reduction is comparable to the level achieved by anti-hypertensive medication.
High blood pressure affects over five million people in England and, if left untreated, increases risk of heart attack, heart failure, kidney disease, stroke or dementia. Normal blood pressure is around 120/80 mmHg.
Researchers from Northumbria University's Department of Sport, Exercise and Rehabilitation worked with fifteen participants who were displaying early hypertension with blood pressure readings of at least 130/90 mmHg, meaning they were at higher risk of experiencing cardiovascular related problems.
They were told that the study was to investigate the effect of a fruit juice on vascular function and were given either 60ml of a Montmorency cherry concentrate or the same amount of a commercially available fruit-flavoured cordial.
Blood pressure and blood samples were taken before the cherry concentrate was consumed and blood pressure was measured on an hourly basis thereafter. Blood samples and a series of other cardiovascular screening tests were taken again on a regular basis over the following eight hours.
The researchers found that the participants who were given the cherry concentrate saw a peak reduction in their blood pressure of 7 mmHg in the three hours after consuming the drink.
Past studies have shown that a reduction of between 5-6 mmHg over a sustained period has been associated with a 38% reduced risk of stroke and 23% reduced risk of coronary heart disease.
Interestingly, those participants with blood pressure levels at the higher end of the scale saw the most benefit.
The greatest improvement in systolic blood pressure occurred when the phenolic acids, protocatechuic and vanillic, within the cherry concentrate reached their peak levels in the plasma. The researchers believe that these particular compounds are, at least in part, responsible for the reduction.
Lead author and Lecturer in Sport and Exercise Nutrition, Karen Keane, explained: "The majority of cardiovascular disease is caused by risk factors that can be controlled, treated or modified, such as high blood pressure, cholesterol, obesity, tobacco use, lack of physical activity and diabetes. Raised blood pressure is the leading cause of deaths from cardiovascular disease, yet relatively small reductions in blood pressure can have a large impact on mortality rates.
"The magnitude of the blood pressure lowering effects we observed was comparable to those achieved by a single anti-hypertensive drug and highlights the potential importance that Montmorency cherries could have in the effective management of high blood pressure."
Drinking tart Montmorency cherry juice can reduce early signs of hypertension: Drinking tart Montmorency cherry juice significantly reduces high blood pressure at a level comparable to that achieved by medication, according to new research from Northumbria University, Newcastle.







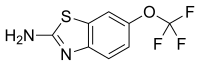 riluzole
riluzole
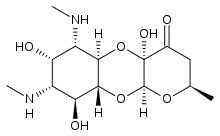
 Apramycin
Apramycin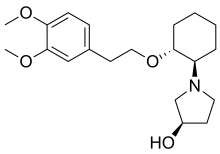 Vernakalant
Vernakalant  Ibutilide
Ibutilide