The U.S. Food and Drug Administration today approved Probuphine, the first buprenorphine implant for the maintenance treatment of opioid dependence. Probuphine is designed to provide a constant, low-level dose of buprenorphine for six months in patients who are already stable on low-to-moderate doses of other forms of buprenorphine, as part of a complete treatment program.
Until today, buprenorphine for the treatment of opioid dependence was only approved as a pill or a film placed under the tongue or on the inside of a person's cheek until it dissolved. While effective, a pill or film may be lost, forgotten or stolen. However, as an implant, Probuphine provides a new treatment option for people in recovery who may value the unique benefits of a six-month implant compared to other forms of buprenorphine, such as the possibility of improved patient convenience from not needing to take medication on a daily basis. An independent FDA advisory committee supported the approval of Probuphine in a meeting held earlier this year.
"Opioid abuse and addiction have taken a devastating toll on American families. We must do everything we can to make new, innovative treatment options available that can help patients regain control over their lives," said FDA Commissioner Robert M. Califf, M.D. "Today's approval provides the first-ever implantable option to support patients' efforts to maintain treatment as part of their overall recovery program."
Expanding the use and availability of medication-assisted treatment (MAT) options like buprenorphine is an important component of the FDA's opioid action plan and one of three top priorities for the U.S. Department of Health and Human Services' Opioid Initiative aimed at reducing prescription opioid and heroin related overdose, death and dependence.
Opioid dependence is the diagnostic term used for the more common concept, "addiction," in the Probuphine clinical trials. Addiction is defined as a cluster of behavioral, cognitive and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use, persisting in drug use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, as well as the possibility of the development of tolerance or development of physical dependence. Physical dependence is not the same as addiction. Newer diagnostic terminology uses the term "opioid use disorder," which includes both milder forms of problematic opioid use as well as addiction.
MAT is a comprehensive approach that combines approved medications (currently, methadone, buprenorphine or naltrexone) with counseling and other behavioral therapies to treat patients with opioid use disorder. Regular adherence to MAT with buprenorphine reduces opioid withdrawal symptoms and the desire to use, without causing the cycle of highs and lows associated with opioid misuse or abuse. At sufficient doses, it also decreases the pleasurable effects of other opioids, making continued opioid abuse less attractive. According to the Substance Abuse and Mental Health Services Administration, patients receiving MAT for their opioid use disorder cut their risk of death from all causes in half.
"Scientific evidence suggests that maintenance treatment with these medications in the context of behavioral treatment and recovery support are more effective in the treatment of opioid use disorder than short-term detoxification programs aimed at abstinence," said Nora Volkow, M.D., director of the National Institute on Drug Abuse at the National Institutes of Health. "This product will expand the treatment alternatives available to people suffering from an opioid use disorder."
Probuphine should be used as part of a complete treatment program that includes counseling and psychosocial support. Probuphine consists of four, one-inch-long rods that are implanted under the skin on the inside of the upper arm and provide treatment for six months. Administering Probuphine requires specific training because it must be surgically inserted and removed. Only a health care provider who has completed the training and become certified through a restricted program called the Probuphine Risk Evaluation and Mitigation Strategy (REMS) program should insert and remove the implants. If further treatment is needed, new implants may be inserted in the opposite arm for one additional course of treatment. The FDA is requiring postmarketing studies to establish the safety and feasibility of placing the Probuphine implants for additional courses of treatment.
The safety and efficacy of Probuphine were demonstrated in a randomized clinical trial of adults who met the clinical criteria for opioid dependence and were considered stable after prior buprenorphine treatment. A response to MAT was measured by urine screening and self-reporting of illicit opioid use during the six month treatment period. Sixty-three percent of Probuphine-treated patients had no evidence of illicit opioid use throughout the six months of treatment – similar to the 64 percent of those who responded to sublingual (under the tongue) buprenorphine alone.
The most common side effects from treatment with Probuphine include implant-site pain, itching, and redness, as well as headache, depression, constipation, nausea, vomiting, back pain, toothache and oropharyngeal pain. The safety and efficacy of Probuphine have not been established in children or adolescents less than 16 years of age. Clinical studies of Probuphine did not include participants over the age of 65.
Probuphine has a boxed warning that provides important safety information for health care professionals, including a warning that insertion and removal of Probuphine are associated with the risk of implant migration, protrusion, expulsion and nerve damage resulting from the procedure. Probuphine must be prescribed and dispensed according to the Probuphine REMS program because of the risks of surgical complications and because of the risks of accidental overdose, misuse and abuse if an implant comes out or protrudes from the skin. As part of this program, Probuphine can only be prescribed and dispensed by health care providers who are certified with the REMS program and have completed live training, among other requirements.
Probuphine implants contain a significant amount of drug that can potentially be expelled or removed, resulting in the potential for accidental exposure or intentional misuse and abuse if the implant comes out of the skin. Patients should be seen during the first week after insertion and a visit schedule of no less than once-monthly is recommended for continued counseling and psychosocial support.
First buprenorphine implant for opioid dependence treatment gets FDA approval: The U.S. Food and Drug Administration today approved Probuphine, the first buprenorphine implant for the maintenance treatment of opioid dependence. Probuphine is designed to provide a constant, low-level dose of buprenorphine for six months in patients who are already stable on low-to-moderate doses of other forms of buprenorphine, as part of a complete treatment program.





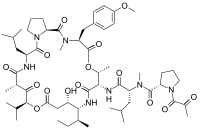
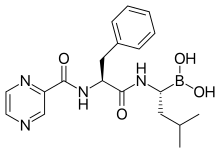
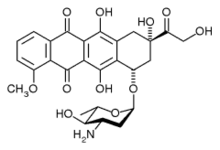 epirubicin
epirubicin letrozole
letrozole 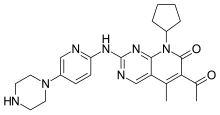 palbociclib
palbociclib