If Dr. Kalipada Pahan's research pans out, the standard advice for failing students might one day be: Study harder and eat your cinnamon!

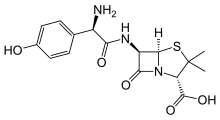
 (sodium benzoate)
(sodium benzoate)
Pahan a researcher at Rush University and the Jesse Brown Veterans Affairs Medical Center in Chicago, has found that cinnamon turns poor learners into good ones--among mice, that is. He hopes the same will hold true for people.
His group published their latest findings online June 24, 2016, in the Journal of Neuroimmune Pharmacology.
"The increase in learning in poor-learning mice after cinnamon treatment was significant," says Pahan. "For example, poor-learning mice took about 150 seconds to find the right hole in the Barnes maze test. On the other hand, after one month of cinnamon treatment, poor-learning mice were finding the right hole within 60 seconds."
Pahan's research shows that the effect appears to be due mainly to sodium benzoate--a chemical produced as cinnamon is broken down in the body.
If that chemical sounds familiar, you may have noticed it on the ingredient labels of many processed foods. Food makers use a synthetic form of it as a preservative. It is also an FDA-approved drug used to treat hyperammonemia--too much ammonia in the blood.
Though some health concerns exist regarding sodium benzoate, most experts agree it's perfectly safe in the amounts generally consumed. One reassuring point is that it's water-soluble and easily excreted in the urine.
Cinnamon acts as a slow-release form of sodium benzoate, says Pahan. His lab studies show that different compounds within cinnamon--including cinnamaldehyde, which gives the spice is distinctive flavor and aroma--are "metabolized into sodium benzoate in the liver. Sodium benzoate then becomes the active compound, which readily enters the brain and stimulates hippocampal plasticity."
Those changes in the hippocampus--the brain's main memory center--appear to be the mechanism by which cinnamon and sodium benzoate exert their benefits.
In their study, Pahan's group first tested mice in mazes to separate the good and poor learners. Good learners made fewer wrong turns and took less time to find food.
In analyzing baseline disparities between the good and poor learners, Pahan's team found differences in two brain proteins. The gap was all but erased when cinnamon was given.
"Little is known about the changes that occur in the brains of poor learners," says Pahan. "We saw increases in GABRA5 and a decrease in CREB in the hippocampus of poor learners. Interestingly, these particular changes were reversed by one month of cinnamon treatment."
The researchers also examined brain cells taken from the mice. They found that sodium benzoate enhanced the structural integrity of the cells--namely in the dendrites, the tree-like extensions of neurons that enable them to communicate with other brain cells.
Cinnamon, like many spices, has antioxidant and anti-inflammatory properties. So it could be expected to exert a range of health-boosting actions, and it does have a centuries-long history of medicinal use around the world.
But the U.S. National Center for Complementary and Integrative Health says that "high-quality clinical evidence to support the use of cinnamon for any medical condition is generally lacking." Most of the clinical trials that have taken place have focused on the spice's possible effect on blood sugar for people with diabetes. Little if any clinical research has been done on the spice's possible brain-boosting properties.
Pahan hopes to change that. Based on the promising results from his group's preclinical studies, he believes that "besides general memory improvement, cinnamon may target Alzheimer's disease, mild cognitive impairment [a precursor to Alzheimer's], and Parkinson's disease as well." He is now talking with neurologists about planning a clinical trial on Alzheimer's.
Before you start heaping cinnamon on your oatmeal, keep a few caveats in mind.
First, most cinnamon found in the store is the Chinese variety, which contains a compound called coumarin that may be toxic to the liver in high amounts. A person would likely have to eat tons of cinnamon to run into a problem, but just the same, Pahan recommends the Ceylon or Sri Lanka type, which is coumarin-free.
Even then, don't overdo it. "Anything in excess is toxic," says Pahan.
What about simply inhaling the pleasant-smelling spice? Will that benefit the brain?
"Simply smelling the spice may not help because cinnamaldehyde should be metabolized into cinnamic acid and then sodium benzoate," explains Pahan. "For metabolism [to occur], cinnamaldehyde should be within the cell."
As for himself, Pahan isn't waiting for clinical trials. He takes about a teaspoonful--about 3.5 grams--of cinnamon powder mixed with honey as a supplement every night.
Should the research on cinnamon continue to move forward, he envisions a similar remedy being adopted by struggling students worldwide.
"Individual differences in learning and educational performance is a global issue, he says. "In many cases, we find two students of the same background studying in the same class, and one turns out to be a poor learner and does worse than the other academically. Now we need to find a way to test this approach in poor learners. If these results are replicated in poor-learning students, it would be a remarkable advance. At present, we are not using any other spice or natural substance."




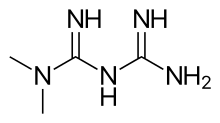 Metformin
Metformin





 curcumin
curcumin silymarin
silymarin