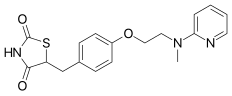
https://en.wikipedia.org/wiki/Rosiglitazone

We've tested our systems in-vitro on stem and tumor cells. Stem cells were used as a model of healthy cells in the experiment and tumor cells as a model of diseased cells. As a result, the anti-tumor drug affected tumor cells as they were irradiated with a laser, and almost no toxicity was observed in healthy cells. The control cells also survived the experiment, which means that tumor cells died as a result of the drug release. This is how we created efficient light-sensitive systems for optically driven drug delivery."Mikhail V. Zyuzin, researcher at ITMO's Faculty of Physics and Engineering
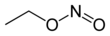

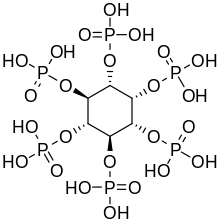
"Calcification occurs when calcium phosphate crystals are deposited in tissue," explains Jean-Christophe Leroux, professor of drug formulation and delivery at ETH Zurich. "The compound adheres to calcium phosphate crystals, inhibiting their growth."
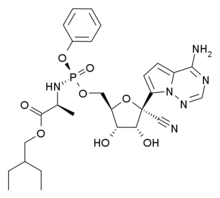


The drug cabotegravir is given every two months. It was 89 percent more effective at preventing HIV infection than Truvada pills, but both reduced the risk, the Associated Press reported. The study, which took place in Africa, was stopped early due to the promising results. The new findings echo those announced earlier this year from a study that compared the shots against the daily pills in gay men, the AP reported.
EMD Serono, the healthcare business sector of Merck KGaA, Darmstadt, Germany in the US and Canada, announced that the US Food and Drug Administration (FDA) has approved Tepmetko (tepotinib) following Priority Review for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
The approval is based on results from the pivotal Phase II VISION study evaluating Tepmetko as monotherapy in patients with advanced NSCLC with METex14 skipping alterations.
"METex14 skipping occurs in approximately 3% to 4% of NSCLC cases, and patients with this aggressive lung cancer are often elderly and face a poor clinical prognosis," said Paul K. Paik, M.D., VISION primary investigator and Clinical Director, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center. "There is a pressing need for targeted treatments that have the potential to generate durable anti-tumor activity and improve the lives of patients with this challenging disease. Tepmetko offers an important and welcome new therapeutic option for patients with metastatic NSCLC harboring these genetic mutations."
"In recent years, the treatment of lung cancer has seen powerful progress in the understanding of the genetic mutations that lead to tumor growth, resistance and progression," said Andrea Ferris, President and CEO of LUNGevity. "The availability of a new precision medicine for NSCLC with METex14 skipping alterations advances patient access to targeted treatment and underscores the importance of routine comprehensive biomarker testing for patients with this challenging cancer."
Tepmetko is the first and only FDA approved MET inhibitor that offers once-daily oral dosing and is administered as two 225 mg tablets (450 mg). Patients with metastatic NSCLC should be selected for treatment with Tepmetko based on the presence of MET exon 14 skipping alterations.
"This approval of Tepmetko by the FDA is an important milestone on our mission to significantly improve the treatment of cancer where MET plays a driving role," said Danny Bar-Zohar, M.D., Global Head of Development for the Healthcare business of Merck KGaA, Darmstadt, Germany. "Our focus now is to ensure Tepmetko is accessible to patients in the United States and fully integrated into clinical practice given the important advance it represents for indicated patients as an oral once-a-day precision medicine."
EMD Serono, the healthcare business of Merck KGaA, Darmstadt, Germany in the US and Canada, is committed to providing patient access and reimbursement support for eligible Tepmetko patients through its Oncology Navigation Center™ (ONC) program in the US. ONC provides a spectrum of patient access and reimbursement support services intended to help US patients receive appropriate treatment access. ONC may be reached at 1-844-662-3631 (844-ONC-EMD1) between 8am-8pm Eastern Time, Monday through Friday, or by visiting OncNavigationCenter.com.
Tepmetko was the first oral MET inhibitor to receive a regulatory approval anywhere in the world for the treatment of advanced NSCLC harboring MET gene alterations, with its approval in Japan in March 2020. The FDA completed its review of Tepmetko under its Real-Time Oncology Review pilot program after previously granting the medicine Breakthrough Therapy Designation. The FDA also recently granted Tepmetko Orphan Drug Designation (ODD).
A Marketing Authorization Application for tepotinib for a similar indication was validated by the European Medicines Agency in November 2020. Applications have also been submitted in Australia, Switzerland, and Canada under the FDA's Project Orbis initiative, which provides a framework for concurrent submission and review of oncology medicines among international partners.1
In the study, Tepmetko demonstrated an overall response rate of 43% (95% CI, 32–56) in treatment-naïve patients (n=69) and 43% (95% CI, 33-55) in previously treated patients (n=83). Median duration of response (DOR) was 10.8 months (95% CI, 6.9-NE) and 11.1 months (95% CI, 9.5-18.5) among treatment-naïve and previously treated patients, respectively. Duration of response of six months or more occurred among 67% of treatment-naïve patients and 75% of previously treated patients, and duration of response of nine months or more occurred among 30% of treatment-naïve patients and 50% of previously treated patients.3
The safety population included 255 patients with NSCLC positive for METex14 skipping alterations, who received Tepmetko in the VISION study. Fatal adverse reactions occurred in one patient (0.4%) due to pneumonitis, one patient (0.4%) due to hepatic failure, and one patient (0.4%) due to dyspnea from fluid overload. Serious adverse reactions occurred in 45% of patients who received Tepmetko. Serious adverse reactions occurring in >2% of patients included pleural effusion (7%), pneumonia (5%), edema (3.9%), dyspnea (3.9%), general health deterioration (3.5%), pulmonary embolism (2%), and musculoskeletal pain (2%). The most common adverse reactions (≥20%) in patients who received Tepmetko were edema, fatigue, nausea, diarrhea, musculoskeletal pain, and dyspnea.