Tuesday, July 16, 2024
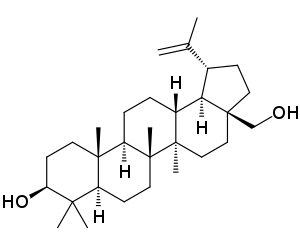
FDA Approves Filsuvez (birch triterpenes) Topical Gel for the Treatment of Epidermolysis Bullosa
FDA Approves Filsuvez () Topical Gel for the Treatment of Epidermolysis Bullosa
EB is a debilitating inherited skin disease that causes a person’s skin to be so fragile it can be injured just from touch. This rare, chronic, and distressing disorder affects infants, children and adults and is intensely painful; recurrent blistering and chronic wounds can result in intolerable pain with limited mobility. Living with EB entails daily challenges to navigate, including slow-healing wounds at risk of infection and painful dressing changes.
Thursday, March 14, 2024
FDA Approves Daybue (trofinetide) for the Treatment of Rett Syndrome
In continuation of my update on trofinetide
Acadia Pharmaceuticals Inc. (Nasdaq: ACAD) announced the U.S. Food and Drug Administration (FDA) approval of Daybue (trofinetide) for the treatment of Rett syndrome in adult and pediatric patients two years of age and older. Daybue is the first and only drug approved for the treatment of Rett syndrome.
“Today marks an important milestone for the Rett community and Acadia. As the first FDA-approved drug for the treatment of Rett syndrome, Daybue now offers the potential to make meaningful differences in the lives of patients and their families who have lacked options to treat the diverse and debilitating array of symptoms caused by Rett syndrome,” said Steve Davis, Acadia’s Chief Executive Officer. “We are grateful to all of the Rett syndrome patients, caregivers, clinical investigators and our employees who have contributed to making today a reality and look forward to getting Daybue to patients as quickly as possible.”
“Rett syndrome is a profoundly debilitating and complex, rare, neurodevelopmental disorder that presents differently across patients and can lead to an array of unpredictable symptoms throughout the course of a patient’s life,” said Jeffrey L. Neul, M.D., Ph.D., Annette Schaffer Eskind Chair and Director, Vanderbilt Kennedy Center, Professor of Pediatrics, Division of Neurology, Pharmacology, and Special Education, Vanderbilt University Medical Center and Phase 3 LAVENDER™ study investigator. “Now, for the first time after decades of clinical research, healthcare providers finally have a treatment option to address a range of core behavioral, communication and physical symptoms for their patients living with Rett syndrome.”
Rett syndrome is a complex, rare, neurodevelopmental disorder typically caused by a genetic mutation on the MECP2 gene.2,3 It is characterized by a period of normal development until six to 18 months of age, followed by significant developmental regression with loss of acquired communication skills and purposeful hand use. Symptoms of Rett syndrome may also include development of hand stereotypies, such as hand wringing and clapping, and gait abnormalities. Rett syndrome is believed to affect 6,000 to 9,000 patients in the U.S., with a diagnosed population of approximately 4,500 U.S. patients.
The FDA approval of Daybue was supported by results from the pivotal Phase 3 LAVENDER study evaluating the efficacy and safety of trofinetide versus placebo in 187 female patients with Rett syndrome five to 20 years of age. In the study, treatment with Daybue demonstrated statistically significant improvement compared to placebo on both co-primary efficacy endpoints, as measured by the change from baseline in Rett Syndrome Behaviour Questionnaire (RSBQ) total score (p=0.018) and the Clinical Global Impression-Improvement (CGI-I) scale score (p=0.003) at week 12. The RSBQ is a caregiver assessment that evaluates a range of symptoms of Rett syndrome including vocalizations, facial expressions, eye gaze, hand movements (or stereotypies), repetitive behaviors, breathing, night-time behaviors and mood. The CGI-I is a global physician assessment of whether a patient has improved or worsened. In the study, the most common side effects were diarrhea (82%) and vomiting (29%).
“This is a historic day for the Rett syndrome community and a meaningful moment for the patients and caregivers who have eagerly awaited the arrival of an approved treatment for this condition,” said Melissa Kennedy, Chief Executive Officer of the International Rett Syndrome Foundation. “Rett syndrome is a complicated, devastating disease that affects not only the individual patient, but whole families. With today’s FDA decision, those impacted by Rett have a promising new treatment option that has demonstrated benefit across a variety of Rett symptoms, including those that impact the daily lives of those living with Rett and their loved ones.”
FDA Approves Daybue (trofinetide) for the Treatment of Rett Syndrome
Monday, March 11, 2024
FDA Approves Zavzpret (zavegepant) Nasal Spray for the Acute Treatment of Migraine
Pfizer Inc. (NYSE: PFE) announced the U.S. Food and Drug Administration (FDA) approval of Zavzpret (zavegepant), the first and only calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray for the acute treatment of migraine with or without aura in adults. In its pivotal Phase 3 study, Zavzpret was statistically superior to placebo on the co-primary endpoints of pain freedom and freedom from most bothersome symptom at two hours post-dose. The pivotal study also demonstrated pain relief as early as 15 minutes in a prespecified secondary endpoint versus placebo.
“The FDA approval of Zavzpret marks a significant breakthrough for people with migraine who need freedom from pain and prefer alternative options to oral medications,” said Angela Hwang, Chief Commercial Officer, President, Global Biopharmaceuticals Business, Pfizer. “Zavzpret underscores Pfizer’s commitment to delivering an additional treatment option to help people with migraine gain relief and get back to their daily lives. Pfizer will continue to build its migraine franchise to further support the billions of people worldwide impacted by this debilitating disease.”
The FDA approval is based on two pivotal randomized, double-blind, placebo-controlled studies that established the efficacy, tolerability and safety profiles of Zavzpret for the acute treatment of migraine. In these studies, Zavzpret was statistically superior to placebo on the co-primary endpoints of pain freedom (defined as a reduction of moderate or severe headache pain to no headache pain) and freedom from most bothersome symptom at two hours post-dose (defined as the absence of the self-identified most bothersome symptom). The pivotal Phase 3 study published in The Lancet Neurology found Zavzpret showed broad efficacy by also demonstrating statistically significant superiority to placebo across 13 of 17 prespecified secondary outcome measures, including early time point endpoints (e.g., 15 and 30-minute pain relief and return to normal function at 30 minutes), return to normal function at 2 hours, and durable efficacy endpoints (e.g., 2-24 and 2-48 hour sustained pain freedom and sustained pain relief). On the 14th endpoint, return to normal function at 15 minutes post-dose, the difference between Zavzpret and placebo was not significant. Consequently, in keeping with the trial’s statistical analysis plan, the remaining secondary endpoints were not formally tested.
“When a migraine hits, it has a significant negative impact on a person’s daily life,” said Kathleen Mullin, M.D., Associate Medical Director at New England Institute for Neurology & Headache. “Among my migraine patients, one of the most important attributes of an acute treatment option is how quickly it works. As a nasal spray with rapid drug absorption, Zavzpret offers an alternative treatment option for people who need pain relief or cannot take oral medications due to nausea or vomiting, so they can get back to normal function quickly.”
Zavzpret was well tolerated in clinical trials. The most common adverse reactions reported in at least 2% of patients treated with Zavzpret and at a frequency greater than placebo were taste disorders (includes dysgeusia and ageusia), nausea, nasal discomfort and vomiting. Zavzpret is contraindicated in patients with a history of hypersensitivity to zavegepant or to any of its components. Hypersensitivity reactions, including facial swelling and urticaria, have occurred with Zavzpret in clinical studies.
https://en.wikipedia.org/wiki/Zavegepant#/media/File:Zavegepant.svg
Friday, March 8, 2024
FDA Approves Skyclarys (omaveloxolone) for the Treatment of Friedreich’s Ataxia
In continuation of my update on omaveloxolone
Reata Pharmaceuticals, Inc. (Nasdaq: RETA) announced the U.S. Food and Drug Administration (“FDA”) approval of Skyclarys™ (omaveloxolone) for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older. With this approval, the FDA granted a rare pediatric disease priority review voucher.
"The approval of Skyclarys, the first therapy specifically indicated for the treatment of Friedreich’s ataxia, is an important milestone for patients affected by this disease as well as their families and caregivers," said Warren Huff, Reata's Chief Executive Officer. "We are grateful to Friedreich’s ataxia patients, investigators, U.S. regulators, and our scientists and employees who made this approval possible. As a company, this is a transformative milestone that highlights our commitment to developing and commercializing novel therapies for patients with severe diseases with few or no approved therapies. We look forward to delivering Skyclarys to eligible patients as quickly as possible."
Friedreich’s ataxia is an ultra-rare, inherited neurodegenerative disorder that is typically diagnosed during adolescence. Patients with Friedreich’s ataxia experience progressive loss of coordination, muscle weakness, and fatigue, which commonly progresses to motor incapacitation and wheelchair reliance by their teens or early twenties, and eventually death. Friedreich’s ataxia affects approximately 5,000 diagnosed patients in the U.S.
“Friedreich's ataxia is a debilitating neuromuscular disease that progressively robs patients of their mobility and independence,” said Susan Perlman, MD, Clinical Professor, Department of Neurology, David Geffen School of Medicine, UCLA. “The approval of Skyclarys represents an important step forward in the treatment of Friedreich's ataxia, providing physicians with the first disease-specific treatment option approved for patients living with this ultra-rare and progressive disease.”
"Today’s approval of Skyclarys represents a significant milestone in our effort to advance research and achieve treatments for Friedreich’s ataxia," said Jen Farmer, Chief Executive Officer at Friedreich's Ataxia Research Alliance. "The entire Friedreich's ataxia community including patients, clinicians, scientists, pharmaceutical companies, government agencies, and others have worked collaboratively for decades to enable therapeutic development for this debilitating disease. Today, we celebrate the impact of an engaged patient community, and we are grateful to the FDA and Reata for working together on the approval of Skyclarys, the first therapy approved in the United States for adult and adolescent patients aged 16 years and older with Friedreich's ataxia.”
The approval of Skyclarys is supported by the efficacy and safety data from the MOXIe Part 2 trial and a post hoc Propensity-Matched Analysis of the open-label MOXIe Extension trial.
MOXIe Part 2 was a randomized, double-blind, placebo-controlled study. Patients with genetically confirmed Friedreich’s ataxia and baseline modified Friedreich’s Ataxia Rating Scale (“mFARS”) scores between 20 and 80 were randomized 1:1 to receive placebo or 150 mg of Skyclarys daily. The primary endpoint was change from baseline in mFARS score compared to placebo at Week 48 in the Full Analysis Population of patients without severe pes cavus (n=82). The mFARS is a clinical assessment tool to assess patient function and is used in clinical trials to assess the efficacy of investigational products for use in Friedreich’s ataxia. Treatment with Skyclarys resulted in statistically significant lower mFARS scores (less impairment) relative to placebo at Week 48. The placebo-corrected difference between the two groups was -2.41 points with a p-value of 0.0138. The most common adverse reactions in MOXIe Part 2 (≥20% and greater than placebo) were elevated liver enzymes (AST/ALT), headache, nausea, abdominal pain, fatigue, diarrhea, and musculoskeletal pain.
Further, in a post hoc Propensity-Matched Analysis, mFARS progression of patients treated with 150 mg of Skyclarys daily in the open-label MOXIe Extension trial was compared to the progression of propensity score-matched untreated patients in the largest natural history study of Friedreich’s ataxia, Clinical Outcome Measures in Friedreich’s ataxia (“FA-COMS”). All patients enrolled in the MOXIe Extension study with at least one post-baseline assessment (n=136) were matched one to one with patients from the FA-COMS study (n=136). Lower (improved) mFARS scores were observed in patients treated with Skyclarys after 3 years relative to the matched set of untreated patients from the FA-COMS natural history study. These exploratory analyses should be interpreted cautiously given the limitations of data collected outside of a controlled study, which may be subject to confounding.
Wednesday, March 6, 2024
FDA Approves Rezzayo (rezafungin for injection) for the Treatment of Candidemia and Invasive Candidiasis
Cidara Therapeutics, Inc. and Melinta Therapeutics, LLC announced the U.S. Food and Drug Administration (FDA) approval of Rezzayo (rezafungin for injection) for the treatment of candidemia and invasive candidiasis in adults with limited or no alternative treatment options. Rezzayo is the first new treatment option approved for patients with candidemia and invasive candidiasis in over a decade.
“The FDA approval of Rezzayo represents a significant milestone for Cidara, and for patients confronted with difficult-to-treat and often deadly candidemia and invasive candidiasis,” said Jeffrey Stein, Ph.D., president and chief executive officer of Cidara. “I am extremely proud of all of the Cidara employees who collectively advanced Rezzayo from preclinical development to NDA approval and am grateful to the many patients and healthcare teams who have participated in the clinical studies.”
George Thompson, M.D., principal investigator in the ReSTORE trial and professor of clinical medicine at the University of California, Davis, School of Medicine, added, “The FDA approval of Rezzayo is tremendous news for those of us who have been hoping for a new option to treat our patients with these deadly fungal infections. Based on the totality of clinical data generated, Rezzayo has the potential to simplify the management of invasive candidiasis and enhance the continuity of echinocandin care.”
The FDA approval of once-weekly Rezzayo was based on clinical data from Cidara’s global ReSTORE Phase 3 trial and supported by the STRIVE Phase 2 clinical trial and extensive non-clinical development program. In clinical studies, Rezzayo, dosed once-weekly, met the FDA and EMA primary endpoints, demonstrating statistical non-inferiority versus caspofungin, a current once-daily standard of care. In addition, overall rates of adverse events and serious adverse events were comparable in patients receiving Rezzayo and caspofungin, while rates of adverse events leading to study drug discontinuation were also similar for Rezzayo and caspofungin. Based on Qualified Infectious Disease Product (QIDP) designation, Rezzayo was approved under Priority Review.
Christine Ann Miller, president and chief executive officer of Melinta Therapeutics, added, “We are thrilled that the FDA has approved Rezzayo, and are firmly committed to offering this innovative therapy to address unmet medical needs and simplify the treatment for patients suffering from invasive Candida infections. We intend to leverage our expansive commercial infrastructure and experience launching anti-infective drugs into acute care settings. We are working closely with Cidara and anticipate bringing Rezzayo, a differentiated once-weekly treatment to patients, this summer.”
Last year, Melinta announced that it had acquired the exclusive rights to commercialize Rezzayo in the U.S. from Cidara. Cidara retains the rights to rezafungin in Japan and has licensed the commercial rights to Melinta Therapeutics in the U.S. and Mundipharma in all other geographies. The European Medicines Agency (EMA) accepted the marketing authorization application (MAA) for rezafungin in August 2022 and it is currently under review.
Monday, March 4, 2024
FDA Approves Brenzavvy (bexagliflozin) for the Treatment of Adults with Type 2 Diabetes
The FDA approval is based on results from a clinical program that evaluated the safety and efficacy of Brenzavvy in 23 clinical trials enrolling more than 5,000 adults with type 2 diabetes mellitus. Phase 3 studies showed Brenzavvy significantly reduced hemoglobin A1c and fasting blood sugar after 24 weeks, either as a monotherapy, in combination with metformin, or as an add-on to standard-of-care treatment consisting of a variety of regimens, including metformin, sulfonylureas, insulin, DPP4 inhibitors, or combinations of these agents. Although Brenzavvy is not approved for weight or blood pressure reduction, modest decreases in both weight and systolic blood pressure have been observed in the clinical program.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Dr. Mason Freeman, M.D., Director of the Translational Research Center at Massachusetts General Hospital. “Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
Brenzavvy treatment can be initiated in adults with type 2 diabetes with an estimated glomerular filtration rate (eGFR) greater than 30 mL/min/1.73 m2. Patients with eGFR less than 60 and greater than 30 mL/min/1.73 m2 are said to be in stage 3 chronic kidney disease, and for these patients metformin is often avoided due to the risk of lactic acidosis.
“Today's FDA approval represents a significant milestone for TheracosBio and provides an important treatment option to patients who suffer from type 2 diabetes. We look forward to bringing Brenzavvy to market,” said Albert R. Collinson, Ph.D., President and CEO of TheracosBio. “The approval of the Brenzavvy NDA is a result of the tireless work of the TheracosBio team and investigators. I want to thank all of the patients who took part in our clinical trials.”
According to the U.S. Centers for Disease Control and Prevention, more than 33 million Americans have type 2 diabetes, which means their bodies don’t use insulin correctly and as a result their blood sugar levels are too high. While some people can control their blood sugar levels with exercise and a healthy diet, others may need additional help to achieve good blood sugar (glycemic) control.
SGLT2 inhibitors are a class of prescription medicines that lower blood sugar by causing the kidneys to remove sugar from the body through urine.
Brenzavvy is available as 20 mg oral tablets recommended to be taken once daily, in the morning with or without food.
Friday, March 1, 2024
FDA Approves Jaypirca (pirtobrutinib) for Adult Patients with Relapsed or Refractory Mantle Cell Lymphoma
Jaypirca, a highly selective kinase inhibitor, utilizes a novel binding mechanism and is the first and only FDA approved non-covalent (reversible) BTK inhibitor. Jaypirca can reestablish BTK inhibition in MCL patients previously treated with a covalent BTK inhibitor (ibrutinib, acalabrutinib, or zanubrutinib) and extend the benefit of targeting the BTK pathway.
"The approval of Jaypirca represents an important advance for patients with relapsed or refractory MCL, who currently have limited options and historically have had a poor prognosis following discontinuation of treatment with a covalent BTK inhibitor," said Michael Wang, M.D., Puddin Clarke Endowed Professor of Lymphoma and Myeloma at The University of Texas MD Anderson Cancer Center. "These data indicate that Jaypirca can provide efficacy in patients previously treated with a covalent BTK inhibitor, potentially extending the time patients may benefit from BTK inhibition therapy. Jaypirca offers a new approach to targeting the BTK pathway following treatment with a covalent BTK inhibitor and has the potential to meaningfully impact the treatment paradigm for relapsed and refractory MCL patients."
The labeling for Jaypirca contains warnings and precautions for infections, hemorrhage, cytopenias, atrial fibrillation and flutter, second primary malignancies, and embryo-fetal toxicity. See Important Safety Information below and full Prescribing Information for additional information, including dosing modifications.
"We are pleased to bring a meaningful new therapeutic option to patients with MCL that can reestablish the benefit of targeting the BTK pathway after receiving multiple prior therapies, including a covalent BTK inhibitor," said Jacob Van Naarden, chief executive officer, Loxo@Lilly. "We are grateful to the patients, investigators, and other members of the clinical care teams for their contributions. Our team has been committed to rapidly advancing the development of Jaypirca for patients with MCL, and we look forward to building on this milestone by continuing to bring forward important new treatments for people with hematologic malignancies."
The FDA approval is based on data from a subset of patients in the BRUIN Phase 1/2 trial. The assessment of efficacy was based on 120 patients with MCL treated with Jaypirca 200 mg once daily until disease progression or unacceptable toxicity. Patients with active central nervous system lymphoma or allogeneic hematopoietic stem cell transplantation or CAR T-cell therapy within 60 days were excluded. Patients had received a median of three prior lines of therapy (range: 1 to 9), with 93% having two or more prior lines; all patients received one or more prior lines of therapy containing a covalent BTK inhibitor. Eighty-three percent (83%) of patients discontinued their last BTK inhibitor due to refractory or progressive disease. Efficacy was based on overall response rate (ORR) and duration of response (DOR) as assessed by an independent review committee (IRC) using 2014 Lugano criteria.
Thursday, February 29, 2024
FDA Approves Orserdu (elacestrant) for Patients with ESR1 Mutations in ER+, HER2- Advanced or Metastatic Breast Cancer
“The FDA approval of Orserdu marks the first ever therapy for ER+, HER2- advanced or metastatic breast cancer patients with ESR1 mutations and we are very proud to offer a targeted therapy addressing this huge unmet need,” commented Elcin Barker Ergun, Chief Executive Officer of the Menarini Group. “We are grateful to the patients, investigators and administrators who participated in the clinical trials that led to this remarkable innovation.”
Orserdu is approved under the FDA’s Priority Review and Fast Track designation based on the results of the registrational Phase III trial EMERALD, that demonstrated statistically significant progression-free survival (PFS) with elacestrant vs SOC endocrine monotherapy (fulvestrant, letrozole, anastrozole, exemestane), meeting both primary endpoints in all patients and in those patients whose tumors harbor ESR1 mutations.
In the group of patients whose tumors had ESR1 mutations, elacestrant reduced the risk of progression or death by 45% (PFS HR=0.55, 95% CI: 0.39, 0.77) vs SOC. A post-hoc analysis of the PFS results based on the duration of prior CDK4/6i inhibitors (CDK4/6i) usage was presented at San Antonio Breast Cancer Symposium (SABCS) in December 2022. The median PFS was 8.6 months on elacestrant vs 1.9 months for SOC, in those patients whose tumors harbored ESR1 mutations and had been treated with a CDK4/6i for at least 12 months.
Safety data is consistent with the other endocrine therapies. Most of the adverse events (AEs), including nausea and musculoskeletal pain were grade 1 and 2. No hematological safety signal was observed and none of the patients in either of the two treatment arms had sinus bradycardia.
“Advanced or metastatic ER+, HER2- breast cancer pre-treated with endocrine-based therapy remains an area of unmet medical need. The last endocrine therapy approved was about 20 years ago, and effective endocrine options for this patient population are needed,” said Dr. Aditya Bardia, MD, MPH, Director of Breast Cancer Research at Mass General Cancer Center, Associate Professor at the Medicine Department at Harvard Medical School, and Principal Investigator for the EMERALD trial. “ESR1 mutations are a known driver of resistance to standard endocrine therapy, and so far, have been difficult to treat. The approval of elacestrant is welcomed as it offers a novel option for patients with ER+, HER2- metastatic breast cancer. This therapy targets the ESR1 mutations in metastatic breast cancer and provides patients with a convenient oral once-daily dose.”
“Each year 300,000 Americans are diagnosed with breast cancer and metastatic breast cancer causes the vast majority of deaths from the disease: more than 43,000 annually. We urgently need new and better treatment options to extend and improve the lives of people with metastatic breast cancer,” said Sonya Negley, Executive Director, Metavivor. “We are thrilled to see the approval of Orserdu, a new oral endocrine therapy, for patients who have tumors that harbor ESR1 mutations, which are present in up to 40% of ER+, HER2- advanced or metastatic breast cancer. We advise patients to get tested for ESR1 mutations at progression in their metastatic treatment, so that their healthcare team can identify the right treatment options for their disease.“
Tuesday, February 27, 2024
FDA Approves Jesduvroq (daprodustat) for Anemia Caused by Chronic Kidney Disease for Adults on Dialysis
GSK plc (LSE/NYSE: GSK) announced the US Food and Drug Administration (FDA) approval of Jesduvroq (daprodustat), an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), for the once-a-day treatment of anemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least four months. Jesduvroq is the first innovative medicine for anemia treatment in over 30 years and the only HIF-PHI approved in the US, providing a new oral, convenient option for patients in the US with anemia of CKD on dialysis.
The FDA approval is based on results from the ASCEND-D trial, assessing the efficacy and safety of Jesduvroq for the treatment of anemia of CKD in patients on dialysis. Results were published in the New England Journal of Medicine with additional results published in the New England Journal of Medicine supplementary appendix.
The Safety Information for Jesduvroq includes a boxed warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access. Jesduvroq increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE). Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels. No trial has identified a hemoglobin target level, dose of Jesduvroq, or dosing strategy that does not increase these risks. Use the lowest dose of Jesduvroq sufficient to reduce the need for red blood cell transfusions. Jesduvroq has not been shown to improve quality of life, fatigue, or patient well-being. Jesduvroq is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia or for treatment of anemia of chronic kidney disease in patients who are not on dialysis.
CKD is an increasing global health burden affecting 700 million patients worldwide, with an estimated one in seven patients also developing anemia.1,2 When left untreated or undertreated, anemia of CKD is associated with poor clinical outcomes and leads to a substantial burden on patients and healthcare systems.3 There is an unmet need for oral treatment options with efficacy and safety comparable to current treatments.
LaVarne Burton, President and Chief Executive Officer, American Kidney Fund, said: “Anemia of CKD can be a debilitating condition that is challenging to manage. This news means that patients on dialysis who are living with anemia of CKD now have another treatment option to help manage their anemia.”
A marketing authorisation application for daprodustat is currently under review with the European Medicines Agency, with a regulatory decision anticipated in the first half of 2023. In June 2020, daprodustat tablets were approved by Japan’s Ministry of Health, Labour and Welfare for the treatment of patients with anemia of CKD. In Japan, the brand name for daprodustat is Duvroq, where it is the market leader and preferred HIF-PHI.
Tony Wood, President and Chief Scientific Officer, GSK, said: “Over the last several decades, there has been little innovation in anemia of CKD. We are proud to have developed Jesduvroq as a new oral treatment where there is a patient desire for more options.”
The FDA approval is based on results from the ASCEND-D trial, assessing the efficacy and safety of Jesduvroq for the treatment of anemia of CKD in patients on dialysis. Results were published in the New England Journal of Medicine with additional results published in the New England Journal of Medicine supplementary appendix.
The Safety Information for Jesduvroq includes a boxed warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access. Jesduvroq increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE). Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels. No trial has identified a hemoglobin target level, dose of Jesduvroq, or dosing strategy that does not increase these risks. Use the lowest dose of Jesduvroq sufficient to reduce the need for red blood cell transfusions. Jesduvroq has not been shown to improve quality of life, fatigue, or patient well-being. Jesduvroq is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia or for treatment of anemia of chronic kidney disease in patients who are not on dialysis.
CKD is an increasing global health burden affecting 700 million patients worldwide, with an estimated one in seven patients also developing anemia.1,2 When left untreated or undertreated, anemia of CKD is associated with poor clinical outcomes and leads to a substantial burden on patients and healthcare systems.3 There is an unmet need for oral treatment options with efficacy and safety comparable to current treatments.
LaVarne Burton, President and Chief Executive Officer, American Kidney Fund, said: “Anemia of CKD can be a debilitating condition that is challenging to manage. This news means that patients on dialysis who are living with anemia of CKD now have another treatment option to help manage their anemia.”
A marketing authorisation application for daprodustat is currently under review with the European Medicines Agency, with a regulatory decision anticipated in the first half of 2023. In June 2020, daprodustat tablets were approved by Japan’s Ministry of Health, Labour and Welfare for the treatment of patients with anemia of CKD. In Japan, the brand name for daprodustat is Duvroq, where it is the market leader and preferred HIF-PHI.
The FDA approval is based on results from the ASCEND-D trial, assessing the efficacy and safety of Jesduvroq for the treatment of anemia of CKD in patients on dialysis. Results were published in the New England Journal of Medicine with additional results published in the New England Journal of Medicine supplementary appendix.
The Safety Information for Jesduvroq includes a boxed warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access. Jesduvroq increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE). Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels. No trial has identified a hemoglobin target level, dose of Jesduvroq, or dosing strategy that does not increase these risks. Use the lowest dose of Jesduvroq sufficient to reduce the need for red blood cell transfusions. Jesduvroq has not been shown to improve quality of life, fatigue, or patient well-being. Jesduvroq is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia or for treatment of anemia of chronic kidney disease in patients who are not on dialysis.
CKD is an increasing global health burden affecting 700 million patients worldwide, with an estimated one in seven patients also developing anemia.1,2 When left untreated or undertreated, anemia of CKD is associated with poor clinical outcomes and leads to a substantial burden on patients and healthcare systems.3 There is an unmet need for oral treatment options with efficacy and safety comparable to current treatments.
LaVarne Burton, President and Chief Executive Officer, American Kidney Fund, said: “Anemia of CKD can be a debilitating condition that is challenging to manage. This news means that patients on dialysis who are living with anemia of CKD now have another treatment option to help manage their anemia.”
A marketing authorisation application for daprodustat is currently under review with the European Medicines Agency, with a regulatory decision anticipated in the first half of 2023. In June 2020, daprodustat tablets were approved by Japan’s Ministry of Health, Labour and Welfare for the treatment of patients with anemia of CKD. In Japan, the brand name for daprodustat is Duvroq, where it is the market leader and preferred HIF-PHI.
https://en.wikipedia.org/wiki/Daprodustat
https://go.drugbank.com/drugs/DB11682
Saturday, February 24, 2024
FDA Approves Miebo (perfluorohexyloctane) Ophthalmic Solution for the Treatment of the Signs and Symptoms of Dry Eye Disease
Bausch + Lomb Corporation, announced that the U.S. Food and Drug Administration (FDA) has approved Miebo™ (perfluorohexyloctane ophthalmic solution; formerly known as NOV03), for the treatment of the signs and symptoms of dry eye disease (DED). Miebo is the first and only FDA-approved treatment for DED that directly targets tear evaporation.
“Today’s FDA approval of Miebo further advances DED treatment by addressing a significant unmet need for millions of people suffering with this disease,” said Brent Saunders, chairman and CEO, Bausch + Lomb. “We are proud to bring to market the first and only prescription eye drop approved in the United States for the treatment of DED that directly targets evaporation. We expect to make Miebo commercially available in the second half of this year.”
DED affects millions of Americans and is one of the most common ocular surface disorders.1 A leading cause of DED is excessive tear evaporation, which due to an altered tear lipid layer, is often associated with the clinical signs of Meibomian gland dysfunction (MGD). An unstable tear film triggers increased ocular surface desiccation, inflammation and damage to the ocular surface.2,3 Miebo is designed to reduce tear evaporation at the ocular surface.4,5
In GOBI and MOJAVE, two phase 3 pivotal clinical trials which enrolled more than 1,200 patients (randomized 1:1 to Miebo or hypotonic saline) with a history of DED and clinical signs of MGD, Miebo consistently met its primary clinical sign and patient-reported symptom endpoint.
"In the two pivotal clinical trials, Miebo addressed the persistent and chronic nature of DED by providing sustained improvement in both the signs and symptoms of DED,” said Preeya Gupta, M.D., cornea and cataract surgeon, Triangle Eye Consultants, Raleigh, North Carolina. “Because Miebo inhibits evaporation, it may be an appropriate treatment option for patients whose tear evaporation exceeds tear supply.”
“Tear evaporation, which is a leading driver of DED, presents a significant treatment challenge. With the approval of Miebo, eye care professionals can now take a new approach to DED therapy with a first-in-class water- and preservative-free prescription treatment option that specifically addresses tear evaporation,” said Paul Karpecki, O.D., director, Cornea and External Disease, Kentucky Eye Institute, and associate professor, University of Pikeville, Kentucky College of Optometry.
Ref : Read More
Thursday, February 22, 2024
FDA Approves Lumryz (sodium oxybate) for Cataplexy or Excessive Daytime Sleepiness in Adults with Narcolepsy
In continuation of my update on Lumryz
Avadel Pharmaceuticals plc a biopharmaceutical company announced the U.S. Food & Drug Administration (FDA) final approval to Lumryz, an extended-release formulation of sodium oxybate indicated to be taken once at bedtime for the treatment of cataplexy or excessive daytime sleepiness (EDS) in adults with narcolepsy. With final approval, Lumryz becomes the first and only FDA approved once-at-bedtime oxybate for people living with narcolepsy. Lumryz was additionally granted Orphan Drug Exclusivity by the FDA.
“Today’s landmark approval and receipt of Orphan Drug Exclusivity represents a major milestone for both Avadel and people living with narcolepsy. As we have heard from key stakeholders, previously approved narcolepsy therapies have the potential to disrupt sleep by either causing insomnia or through forced awakening during the middle of the night for their crucial second dose. Lumryz can now offer people with narcolepsy the opportunity for an uninterrupted night sleep while receiving the full benefit of their prescribed treatment in one single bedtime dose that addresses their symptoms of narcolepsy,” said Greg Divis, Chief Executive Officer of Avadel. “We would like to thank the patients, caregivers, clinical trial investigators, healthcare providers, and advocates who have tirelessly partnered with us throughout the drug development process and look forward to providing the narcolepsy community access to now approved Lumryz.”
Narcolepsy is a chronic neurological condition that impairs the brain's ability to regulate the sleep-wake cycle. The condition affects approximately one in 2,000 people in the United States with the cardinal symptom of EDS. Additional symptoms can vary by person but may include disrupted nighttime sleep, a sudden loss of muscle tone usually triggered by strong emotion (cataplexy), sleep paralysis and hallucinations.
“This long-awaited therapy for people living with narcolepsy fills a critical unmet need by avoiding the burden of a second middle-of-the-night dose that immediate-release oxybate products require. The once-at-bedtime dosing regimen of Lumryz may help restore a more natural sleep-wake cycle,” said Michael J. Thorpy, M.D., an investigator from the REST-ON Phase 3 trial and Director at the Sleep-Wake Disorders Center at Montefiore Medical Center and Professor of Neurology at the Albert Einstein College of Medicine.
The FDA’s final approval of Lumryz was based on positive results from the pivotal Phase 3 REST-ON clinical study completed in March 2020. In the REST-ON Phase 3 trial, once-at-bedtime Lumryz demonstrated highly statistically significant (p<0.001) and clinically meaningful improvement compared to placebo across all three co-primary endpoints (Maintenance of Wakefulness Test, Clinical Global Impression-Improvement and mean weekly cataplexy attacks) for all three doses evaluated, 6, 7.5 and 9 grams.
With this approval, the FDA has also found Lumryz to be clinically superior to currently marketed twice-nightly oxybate products and granted Lumryz seven years of Orphan Drug Exclusivity. In particular, FDA found that Lumryz makes a major contribution to patient care over currently available, twice-nightly oxybate products by providing a once-nightly dosing regimen that avoids nocturnal arousal to take a second dose. The FDA's Orphan Drug program is designed to support the development of drugs that treat a condition affecting less than 200,000 U.S. patients. The seven-year market exclusivity for Lumryz began on the date of FDA approval, May 1, 2023.
“For people living with narcolepsy, and for all of us who advocate for this community, the approval of Lumryz is an important step forward,” said Julie Flygare, JD, President and CEO of Project Sleep. “People living with narcolepsy will finally have a new treatment option to manage EDS and cataplexy, and the fact that this new oxybate option allows for reduced dosing frequency is a game-changing advancement that shows Avadel’s commitment to understanding the patient experience. We look forward to continued collaboration with Avadel as part of a shared mission to positively impact the lives of people with narcolepsy.”
Lumryz has a boxed warning as a central nervous system depressant, and for its potential for abuse and misuse. Lumryz is available only through a restricted program under a Risk Evaluation and Mitigation Strategy called the Lumryz REMS. Most common adverse reactions (incidence > 5% and greater than placebo) reported for all doses of Lumryz combined were nausea, dizziness, enuresis, headache, and vomiting.
Tuesday, February 20, 2024
FDA Approves Mydcombi (tropicamide and phenylephrine hydrochloride) Ophthalmic Spray for Inducing Mydriasis
In continuation of my update on phenylephrine hydrochloride
Eyenovia, Inc, announced the U.S. Food and Drug Administration (FDA) approval of Mydcombi (tropicamide and phenylephrine hydrochloride ophthalmic spray) 1%/2.5% for inducing mydriasis for diagnostic procedures and in conditions where short term pupil dilation is desired. This represents the first approved fixed dose combination of tropicamide and phenylephrine in the United States and also the first product using Eyenovia’s proprietary Optejet device to be approved by any regulatory authority.
Mydcombi is designed to
improve the efficiency of the estimated 106 million office-based comprehensive
eye exams performed every year in the United States, as well as the estimated 4
million pharmacologic mydriasis applications for cataract surgery. The product
is contraindicated and should not be used in patients with known
hypersensitivity to any component of the formulation.
“The approval of Mydcombi, our first FDA approved product,
represents the culmination of years of tireless effort by the entire Eyenovia
team, and I would like to express my sincere gratitude to the associates and
technical experts who helped advance this important program through this
transformational milestone,” stated Michael Rowe, chief executive officer of
Eyenovia. “We look forward to introducing Mydcombi to key offices beginning
this summer while we bring our internal manufacturing capabilities on-line for
2024.”
“Perhaps more importantly, FDA approval of Mydcombi provides
critical validation of the Optejet as it is the first product approved using
the Optejet platform, which is core not only to our internal development
programs, including MicroLine for presbyopia, but our partnered programs as
well. We see opportunities to unlock significant opportunities in the future
treatment of other ophthalmic conditions including glaucoma and dry eye. I am
confident in our ability to maintain our current momentum.”
“I am proud of our team for this significant achievement – which
represents many ‘firsts’ for eye care,” stated Dr. Sean Ianchulev, Founder and
Chairman of Eyenovia’s Board of Directors. “The use of eye dropper bottles has
presented challenges for dosing in ophthalmologic settings in millions of
patients. We can do better now using sophisticated micro-array print delivery
with physiologic dosing that is similar to the natural tear film volume.”
https://en.wikipedia.org/wiki/Phenylephrine
https://en.wikipedia.org/wiki/Tropicamide