Friday, August 23, 2024
UCLA-led research results in FDA approval of 4-drug combination for frontline treatment of metastatic pancreatic cancer
Thursday, August 22, 2024
New treatment for a rare and aggressive cancer improves survival rates in breakthrough clinical trial
Wednesday, August 21, 2024
Ipsen’s Onivyde Regimen, a Potential New Standard-of-Care First-Line Therapy in Metastatic Pancreatic Adenocarcinoma, Approved by FDA
Friday, August 16, 2024
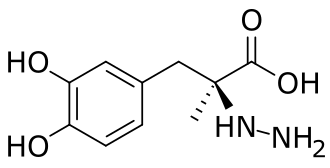
Zevra Therapeutics Announces Resubmission of Arimoclomol New Drug Application to the U.S. Food and Drug Administration
Thursday, August 15, 2024
Zealand Pharma Submits New Drug Application to the US FDA for Glepaglutide in Short Bowel Syndrome
“Short bowel syndrome with intestinal failure is a complex, chronic and severe condition in which individuals are dependent on receiving fluids and nutrition parenterally. While life-sustaining, parenteral support poses significant restrictions on daily life and carries a risk of serious and life-threatening complications. More effective and convenient treatments to further reduce parenteral support are needed, with the ultimate goal of discontinuing parenteral support and achieving enteral autonomy,” said David Kendall, MD, Chief Medical Officer of Zealand Pharma. “We believe glepaglutide, once approved, can reduce both the burden of parenteral support and of daily dosing of existing GLP-2 treatment for people living with SBS and intestinal failure, and we are pleased to submit this treatment for regulatory review and potential approval in the US.”
Wednesday, August 14, 2024
Journey Medical Corporation Submits New Drug Application to FDA for DFD-29 to Treat Rosacea
“This NDA submission is a significant milestone for Journey Medical and we look forward to collaborating with the FDA during its review to bring DFD-29, a potentially differentiated, best-in-class oral rosacea treatment, one step closer to patients. Based on the data seen in our pivotal trials, DFD-29 could fundamentally improve the treatment paradigm for patients suffering from both inflammatory lesions and erythema (redness) from rosacea,” said Claude Maraoui, Co-Founder, President and Chief Executive Officer of Journey Medical.
Tuesday, August 13, 2024
FDA Grants Soligenix Fast Track Designation for Dusquetide in the Treatment of Oral Lesions of Behçet's Disease
Monday, August 12, 2024
FDA Approves Augtyro (repotrectinib) for the Treatment of Locally Advanced or Metastatic ROS1-Positive Non-Small Cell Lung Cancer (NSCLC)
Bristol Myers Squibb (NYSE: BMY) announced the U.S. Food and Drug Administration (FDA) approval of Augtyro (repotrectinib) for the treatment of adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer (NSCLC). Administered as an oral therapy, Augtyro is a tyrosine kinase inhibitor (TKI) targeting ROS1 oncogenic fusions.
The approval is based on the TRIDENT-1 study, an open-label, single-arm, Phase 1/2 trial that evaluated Augtyro in TKI-naïve and TKI-pretreated patients.2 In TKI-naïve patients (n=71), the primary endpoint of objective response rate (ORR), defined as the percentage of people treated within a certain period of time whose tumor size decreased (partial response) or who no longer have signs of cancer (complete response),was 79% (95% Confidence Interval [CI]: 68 to 88).1,3 The median duration of response (mDOR) was 34.1 months. Among patients pretreated with one prior ROS1 TKI and no prior chemotherapy (n=56), the ORR was 38% (95% CI: 25 to 52) and the mDOR was 14.8 months.1 Among those who had measurable central nervous system (CNS) metastases at baseline, responses in intracranial lesions were observed in 7 of 8 TKI-naïve patients (n=71) and 5 of 12 of those who were TKI-pretreated (n=56).
“New treatment options continue to be needed for patients with ROS1 fusion-positive NSCLC that support important clinical goals, including achieving durable therapeutic responses,” said Jessica J. Lin, MD, TRIDENT-1 primary investigator and attending physician at the Center for Thoracic Cancers at Massachusetts General Hospital and Assistant Professor of Medicine at Harvard Medical School.4,5,6,7 “Based on the data we have seen in the TRIDENT-1 trial, repotrectinib has the potential to become a new standard of care option for patients with locally advanced or metastatic ROS1 fusion-positive lung cancer.”1
Augtyro is associated with the following Warnings & Precautions: central nervous system (CNS) effects, interstitial lung disease (ILD)/pneumonitis, hepatotoxicity, myalgia with creatine phosphokinase elevation, hyperuricemia, skeletal fractures, and embryo-fetal toxicity.1 Please see Important Safety Information below.
“While progress has been made in the treatment of NSCLC over the past decade, there is still a need to address this particularly difficult-to-treat form of the disease with innovative science and a targeted approach,” said Samit Hirawat, MD, executive vice president, chief medical officer, Global Drug Development, Bristol Myers Squibb.6,7 “As the only approved next-generation TKI for ROS1-positiveNSCLCpatients, Augtyro builds on our legacy of delivering transformational therapies for patients with thoracic cancers.”6,8,9
“ROS1-positive NSCLC patients and their families face a stressful journey because our cancer can be difficult to treat, especially when it spreads to the brain,” said Janet Freeman-Daily, co-founder and president of The ROS1ders, a patient advocacy organization.10 “Today’s approval brings a new treatment option for the ROS1-positive patient community, which gives us hope for more time with loved ones.”
Augtyro is designed to minimize interactions that can lead to certain forms of treatment resistance in ROS1-positive metastatic NSCLC patients. Itis expected to be available to patients in the U.S. in mid-December 2023. Bristol Myers Squibb thanks the patients and investigators involved in the TRIDENT-1 clinical trial program.
ref ;https://en.wikipedia.org/wiki/Repotrectinib
Bridgebio Pharma Announces U.S. Food and Drug Administration (FDA) Acceptance of New Drug Application (NDA) for Acoramidis for the Treatment of Patients with Transthyretin Amyloid Cardiomyopathy (ATTR-CM)
BridgeBio Pharma, Inc. (Nasdaq: BBIO) (“BridgeBio” or the “Company”), a commercial-stage biopharmaceutical company focused on genetic diseases and cancers, today announced that the U.S. Food and Drug Administration (FDA) has accepted for filing the Company’s New Drug Application (NDA) for acoramidis, an investigational drug for the treatment of ATTR-CM. The application was based on positive results from ATTRibute-CM, the Company’s Phase 3 study designed to evaluate the efficacy and safety of acoramidis, an investigational, next-generation, orally-administered, highly potent, small molecule stabilizer of transthyretin (TTR). The FDA has set an action date of November 29, 2024 under the PDUFA. The FDA also notified the Company that it is not currently planning to hold an advisory committee meeting to discuss the application.
“The FDA’s acceptance of our NDA submission for review reinforces our belief in acoramidis and its potential to make an important contribution to the care of patients with ATTR-CM,” said Jonathan Fox, MD, PhD, President and Chief Medical Officer of BridgeBio Cardiorenal. “We look forward to the upcoming review process and the potential for approval in the United States. Similarly, with the European Marketing Authorization Application accepted and with plans to extend our submissions to other countries and regions, we are committed to making acoramidis available to patients.”
In July 2023, BridgeBio announced positive results from ATTRibute-CM, reporting a highly statistically significant result, demonstrated by a Win Ratio of 1.8 (p<0.0001) on the primary endpoint (a hierarchical analysis prioritizing in order: ACM, then frequency of CVH, then change from baseline in N-terminal prohormone of brain natriuretic peptide (NT-proBNP), then change from baseline in 6-minute walk distance (6MWD)). Acoramidis was well-tolerated, with no safety signals of potential clinical concern identified. BridgeBio has also presented analyses from ATTRibute-CM at the European Society of Cardiology Congress 2023 and at the American Heart Association Scientific Sessions 2023.
“As part of our mission, we seek to improve the lives of patients with amyloidosis by providing support to them and their caregivers throughout their journey. There is a need for more treatment options that can help fill the significant unmet need that exists for patients today. We are excited by BridgeBio’s recent NDA acceptance from the FDA, which we hope moves us one step closer to having acoramidis available as a treatment for the ATTR-CM community,” said Isabelle Lousada, president and CEO of the Amyloidosis Research Consortium, a global nonprofit organization dedicated to advancements in amyloidosis.
The Company also received acceptance of its Marketing Authorization Application with the European Medicines Agency and is preparing for additional global regulatory submissions.
Friday, August 9, 2024
Amneal Announces Complete Response Resubmission for IPX203 New Drug Application
“We are pleased to provide our complete response resubmission for IPX203 as we look to expand our Parkinson’s franchise,” said Chirag and Chintu Patel, Co-Chief Executive Officers at Amneal. “We look forward to launching this much-needed treatment in the second half of 2024, subject to FDA approval.”
PD is characterized by slowness of movement, stiffness, resting tremor and impaired balance. While PD is not considered a fatal disease, it is associated with significant morbidity and disability. The average age at diagnosis for patients with PD is 60; as people live longer, the number of patients living with PD is predicted to grow significantly over the coming decades.
Thursday, August 8, 2024
Defender Pharmaceuticals Receives Complete Response Letter from the U.S. Food and Drug Administration for its Intranasal Scopolamine (DPI-386) New Drug Application for the Prevention of Nausea and Vomiting Induced by Motion in Adults
Certain motions cause discomfort in individuals while engaged in various leisure or travel-related activities. Most forms of travel, whether on land, in the air, or on the water, can trigger symptoms such as nausea and vomiting (example: flying, boating/fishing, car, bus, and train). Symptoms induced by motion can also have a detrimental impact on the ability of various military personnel and astronauts to perform assigned duties, potentially impacting readiness and negatively impacting resources. Motion-related discomfort is a common and transient response to unfamiliar or unnatural motion or contradictory spatial sensory information, resulting in decrements to performance of tasks, pallor, cold sweating, nausea and vomiting. Prolonged exposure to certain motions may induce sopite-related symptoms such as loss of drive and concentration, drowsiness, sleepiness, apathy, depression, and a feeling of impending doom.
Wednesday, August 7, 2024
Lykos Therapeutics Announces FDA Acceptance and Priority Review of New Drug Application for MDMA-Assisted Therapy for PTSD
Lykos, with longstanding roots in advocacy for psychedelic medicine, pioneered the first randomized, double-blind, placebo controlled clinical trials evaluating the efficacy and safety of MDMA-assisted therapy as an investigational modality using midomafetamine (MDMA) in combination with psychological intervention to treat PTSD.
With a growing body of evidence supporting the potential medical use of MDMA, in 2017 the FDA granted the company's investigational MDMA-assisted therapy Breakthrough Therapy designation, a process designed to expedite the development and review of drugs intended to treat serious conditions for which preliminary scientific evidence indicates that it may demonstrate a substantial improvement over available therapies. If approved by the FDA, the U.S. Drug Enforcement Administration ("DEA") would be required to reschedule MDMA making it available for prescription medical use.