 Ibrutinib
IbrutinibMonday, August 29, 2016
Janssen gets positive CHMP opinion for IMBRUVICA (ibrutinib) to treat patients with previously untreated CLL
 Ibrutinib
IbrutinibTuesday, September 20, 2016
Ibrutinib: Indication of added benefit in one of three therapeutic indications...

Saturday, August 11, 2018
Researchers find leukemia and lymphoma drug may benefit glioblastoma patients
"Glioblastoma is the most lethal primary brain tumor and is highly resistant to current therapies," said Bao. "There is an urgent need to get new treatments to these patients as quickly as possible."
"Additional research is important to understand the effects of ibrutinib in patients, but these early findings are promising," said Bao. "Using an FDA-approved drug would allow us to surpass many of the lengthy regulatory studies needed when developing a new treatment, and we could potentially begin clinical trials very soon."
Saturday, July 28, 2018
FDA-approved drug for lymphoma and leukemia may help treat common type of brain tumor

"Glioblastoma is the most lethal primary brain tumor and is highly resistant to current therapies," said Bao. "There is an urgent need to get new treatments to these patients as quickly as possible."
"Additional research is important to understand the effects of ibrutinib in patients, but these early findings are promising," said Bao. "Using an FDA-approved drug would allow us to surpass many of the lengthy regulatory studies needed when developing a new treatment, and we could potentially begin clinical trials very soon."
Monday, February 22, 2016
Ibrutinib ‘new standard’ for relapsed, refractory mantle cell lymphoma
 Temsirolimus
Temsirolimus Ibrutinib
IbrutinibTuesday, July 23, 2013
New Drug Application Submitted to U.S. FDA for Ibrutinib in the Treatment of Two B-Cell Malignancies
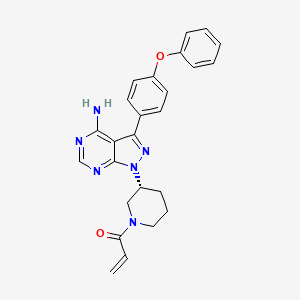
 We know that, Ibrutinib, also known as PCI-32765, is an experimental drug candidate for the treatment of various types of cancer. It is an orally-administered, selective and covalent inhibitor of the enzyme Bruton tyrosine kinase (Btk). Ibrutinib is currently under development by Pharmacyclics, Inc and Johnson & Johnson's Janssen.
We know that, Ibrutinib, also known as PCI-32765, is an experimental drug candidate for the treatment of various types of cancer. It is an orally-administered, selective and covalent inhibitor of the enzyme Bruton tyrosine kinase (Btk). Ibrutinib is currently under development by Pharmacyclics, Inc and Johnson & Johnson's Janssen.Thursday, September 24, 2015
Ibrutinib (IMBRUVICA) improves survival in treatment-naïve patients with chronic lymphocytic leukemia
Wednesday, January 27, 2016
Ibrutinib more effective than traditional chemotherapy in older untreated patients with CLL
Thursday, July 30, 2015
Newly approved drug for rare blood cancer shows sustained benefit for 2 years
We know that, Ibrutinib also known as PCI-32765 and marketed under the name Imbruvica) is an anticancer drug targeting B-cell malignancies. It was approved by the US FDA in November 2013 for the treatment of mantle cell lymphoma and in February 2014 for the treatment of chronic lymphocytic leukemia It is an orally-administered, selective and covalent inhibitor of the enzyme Bruton's tyrosine kinase (BTK) Ibrutinib is currently under development by Pharmacyclics, Inc and Johnson & Johnson'sJanssen Pharmaceutical division for additional B-cell malignancies including diffuse large B-cell lymphoma and multiple myeloma
Thursday, December 17, 2015
IMBRUVICA (ibrutinib) wins Prix Galien USA 2015 Award in Best Pharmaceutical Agent category

Friday, May 20, 2016
RUVICA (ibrutinib) capsules approved for treatment-naïve CLL patients
Saturday, February 9, 2019
FDA Approves Imbruvica (ibrutinib) Plus Obinutuzumab as First Non-Chemotherapy Combination Regimen for Treatment-Naïve Patients with Chronic Lymphocytic Leukemia

Tuesday, May 24, 2016
FDA Approves Imbruvica (ibrutinib) for the First-Line Treatment of Chronic Lymphocytic Leukemia

Thursday, September 17, 2015
Combining targeted drug with chemotherapy offers longer life to b-cell cancer patients
Wednesday, November 14, 2018
FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients

The combination of Imbruvica and rituximab provides health care professionals with a new treatment option for patients living with this serious blood cancer,” said Dr. Lia Palomba, hematologist-oncologist at Memorial Sloan-Kettering Cancer Center, New York, and iNNOVATE study investigator. “Before Imbruvica, there were no FDA-approved treatment options for patients with Waldenström’s macroglobulinemia, a disease first acknowledged nearly 75 years ago. Today, Imbruvica continues to provide an important therapeutic approach in the treatment of this complex disease.”
“Results from iNNOVATE showed significant improvement in progression-free survival at 30 months and demonstrated the superiority of Imbruvica plus rituximab over rituximab monotherapy in Waldenström's macroglobulinemia,” said Meletios A. Dimopoulos, M.D., Professor and Chairman of the Department of Clinical Therapeutics, National and Kapodistrian University of Athens School of Medicine, Athens, Greece, and iNNOVATE lead study investigator. “Based on these results, Imbruvica in combination with rituximab may be considered as a first- and second-line option for appropriate people diagnosed and living with WM.”
“The clinical data generated for Imbruvica plus rituximab in the treatment of Waldenström’s macroglobulinemia offers physicians evidence to consider this combination regimen for newly-diagnosed patients. Today’s approval represents an important milestone for people living with this rare and incurable blood cancer who have limited FDA-approved treatment options,” said Andree Amelsberg, M.D., Vice President of Oncology Medical Affairs at Janssen Scientific Affairs, LLC. “We remain dedicated to a comprehensive clinical development program to explore the full potential of Imbruvica, including in combination with other therapies.”
Friday, March 1, 2024
FDA Approves Jaypirca (pirtobrutinib) for Adult Patients with Relapsed or Refractory Mantle Cell Lymphoma
Jaypirca, a highly selective kinase inhibitor, utilizes a novel binding mechanism and is the first and only FDA approved non-covalent (reversible) BTK inhibitor. Jaypirca can reestablish BTK inhibition in MCL patients previously treated with a covalent BTK inhibitor (ibrutinib, acalabrutinib, or zanubrutinib) and extend the benefit of targeting the BTK pathway.
"The approval of Jaypirca represents an important advance for patients with relapsed or refractory MCL, who currently have limited options and historically have had a poor prognosis following discontinuation of treatment with a covalent BTK inhibitor," said Michael Wang, M.D., Puddin Clarke Endowed Professor of Lymphoma and Myeloma at The University of Texas MD Anderson Cancer Center. "These data indicate that Jaypirca can provide efficacy in patients previously treated with a covalent BTK inhibitor, potentially extending the time patients may benefit from BTK inhibition therapy. Jaypirca offers a new approach to targeting the BTK pathway following treatment with a covalent BTK inhibitor and has the potential to meaningfully impact the treatment paradigm for relapsed and refractory MCL patients."
The labeling for Jaypirca contains warnings and precautions for infections, hemorrhage, cytopenias, atrial fibrillation and flutter, second primary malignancies, and embryo-fetal toxicity. See Important Safety Information below and full Prescribing Information for additional information, including dosing modifications.
"We are pleased to bring a meaningful new therapeutic option to patients with MCL that can reestablish the benefit of targeting the BTK pathway after receiving multiple prior therapies, including a covalent BTK inhibitor," said Jacob Van Naarden, chief executive officer, Loxo@Lilly. "We are grateful to the patients, investigators, and other members of the clinical care teams for their contributions. Our team has been committed to rapidly advancing the development of Jaypirca for patients with MCL, and we look forward to building on this milestone by continuing to bring forward important new treatments for people with hematologic malignancies."
The FDA approval is based on data from a subset of patients in the BRUIN Phase 1/2 trial. The assessment of efficacy was based on 120 patients with MCL treated with Jaypirca 200 mg once daily until disease progression or unacceptable toxicity. Patients with active central nervous system lymphoma or allogeneic hematopoietic stem cell transplantation or CAR T-cell therapy within 60 days were excluded. Patients had received a median of three prior lines of therapy (range: 1 to 9), with 93% having two or more prior lines; all patients received one or more prior lines of therapy containing a covalent BTK inhibitor. Eighty-three percent (83%) of patients discontinued their last BTK inhibitor due to refractory or progressive disease. Efficacy was based on overall response rate (ORR) and duration of response (DOR) as assessed by an independent review committee (IRC) using 2014 Lugano criteria.

