"Our study showed no significant increase in serious adverse events in the combination therapy," said Stephen Peters, M.D., Ph.D., professor of pulmonary, critical care, allergy and immunologic diseases at Wake Forest Baptist Medical Center and lead author of the study.
Thursday, June 15, 2017
Combination drug therapy safe, effective in treating asthma patients
Monday, January 16, 2023
FDA Approves Airsupra (albuterol/budesonide) Metered-Dose Inhaler to Reduce the Risk of Asthma Exacerbations
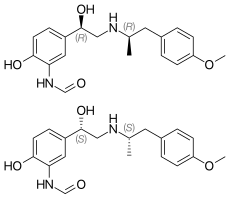
In continuation of my update on albuterol/budesonide
Airsupra (albuterol/budesonide), formerly known as PT027, has been approved in the US for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in people with asthma aged 18 years and older.
The approval by the Food and Drug Administration (FDA) was based on results from the MANDALA and DENALI Phase III trials. In MANDALA, Airsupra significantly reduced the risk of severe exacerbations compared to albuterol in patients with moderate to severe asthma when used as an as-needed rescue medication in response to symptoms. Importantly, in the secondary endpoint of mean annualised total systemic corticosteroid exposure, Airsupra demonstrated a significant reduction compared to albuterol at the approved dose of 180mcg albuterol/160mcg budesonide. In DENALI, Airsupra significantly improved lung function compared to the individual components albuterol and budesonide in patients with mild to moderate asthma.
Airsupra is a first-in-class, pressurised metered-dose inhaler (pMDI), fixed-dose combination rescue medication containing albuterol, a short-acting beta2-agonist (SABA), and budesonide, an anti-inflammatory inhaled corticosteroid (ICS) in the US. It is being developed by AstraZeneca and Avillion.
Bradley E. Chipps, Past President of the American College of Allergy, Asthma & Immunology and Medical Director of Capital Allergy & Respiratory Disease Center in Sacramento, US, said: “People with asthma are at risk of severe exacerbations regardless of their disease severity or level of control. Current albuterol rescue inhalers alleviate acute symptoms, but do not treat the underlying inflammation in asthma. The approval of Airsupra means that for the first time, adults with asthma in the US have a rescue treatment to manage both their symptoms and the inflammatory nature of their disease.”
Mene Pangalos, Executive Vice President, BioPharmaceuticals R&D, AstraZeneca, said: “With patients experiencing more than 10 million asthma exacerbations each year in the US and uncontrolled asthma expected to cost the US economy billions of dollars in direct medical costs alone over the next 20 years, today’s positive decision is good news for those adults with asthma who make up more than 80% of asthma patients in the US. Physicians will be able to offer their patients Airsupra, an important new rescue treatment that reduces the risk of asthma exacerbations.”
Asthma is a chronic, inflammatory respiratory disease with variable symptoms that affects as many as 262 million people worldwide. In the US over 21 million adults have asthma, representing more than 80% of the total number of people with asthma. Adults have 8.5 million exacerbations each year in the US. Uncontrolled asthma will cost the US economy an estimated $300 billion (in 2018 dollar values) in the next 20 years in direct medical costs alone.
The safety and tolerability of Airsupra in both trials were consistent with the known profiles of the components,1,2 with the most common adverse events including headache, oral candidiasis, cough and dysphonia.
Wednesday, September 21, 2011
- The frequency of treatment related adverse events for budesonide MMX 6 mg (21.0%) was similar to placebo (21.3%).
- Mean morning plasma Cortisol levels remained within normal limits at all visits for both budesonide MMX 6 mg and placebo.
- There were no clinically meaningful differences in the numbers of patients with abnormal bone mineral density scans at baseline and end-of-study between budesonide MMX 6 mg and placebo.
"Now that we have the top-line safety data from the extended use study, we are moving forward as planned to submit the NDA in December 2011 for budesonide MMX 9 mg for the induction of remission of mild to moderate active ulcerative colitis," said Gerald T. Proehl, CEO/President of the company...More.: http://ir.santarus.com/releasedetail.cfm?ReleaseID=606515
Thursday, August 1, 2024
FDA Approves Airsupra (albuterol/budesonide) Metered-Dose Inhaler to Reduce the Risk of Asthma Exacerbations
Airsupra (albuterol/budesonide), formerly known as PT027, has been approved in the US for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in people with asthma aged 18 years and older.
The approval by the Food and Drug Administration (FDA) was based on results from the MANDALA and DENALI Phase III trials. In MANDALA, Airsupra significantly reduced the risk of severe exacerbations compared to albuterol in patients with moderate to severe asthma when used as an as-needed rescue medication in response to symptoms. Importantly, in the secondary endpoint of mean annualised total systemic corticosteroid exposure, Airsupra demonstrated a significant reduction compared to albuterol at the approved dose of 180mcg albuterol/160mcg budesonide. In DENALI, Airsupra significantly improved lung function compared to the individual components albuterol and budesonide in patients with mild to moderate asthma.
Bradley E. Chipps, Past President of the American College of Allergy, Asthma & Immunology and Medical Director of Capital Allergy & Respiratory Disease Center in Sacramento, US, said: “People with asthma are at risk of severe exacerbations regardless of their disease severity or level of control. Current albuterol rescue inhalers alleviate acute symptoms, but do not treat the underlying inflammation in asthma. The approval of Airsupra means that for the first time, adults with asthma in the US have a rescue treatment to manage both their symptoms and the inflammatory nature of their disease.”
Mene Pangalos, Executive Vice President, BioPharmaceuticals R&D, AstraZeneca, said: “With patients experiencing more than 10 million asthma exacerbations each year in the US and uncontrolled asthma expected to cost the US economy billions of dollars in direct medical costs alone over the next 20 years, today’s positive decision is good news for those adults with asthma who make up more than 80% of asthma patients in the US. Physicians will be able to offer their patients Airsupra, an important new rescue treatment that reduces the risk of asthma exacerbations.”
Asthma is a chronic, inflammatory respiratory disease with variable symptoms that affects as many as 262 million people worldwide.3 In the US over 21 million adults have asthma, representing more than 80% of the total number of people with asthma.4 Adults have 8.5 million exacerbations each year in the US.4 Uncontrolled asthma will cost the US economy an estimated $300 billion (in 2018 dollar values) in the next 20 years in direct medical costs alone.5
The safety and tolerability of Airsupra in both trials were consistent with the known profiles of the components,1,2 with the most common adverse events including headache, oral candidiasis, cough and dysphonia