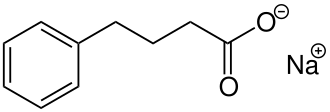
Sodium phenylbutyrate

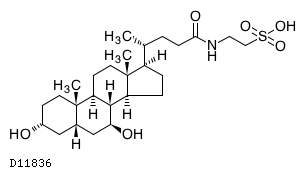
Taurursodiol
Amylyx
Pharmaceuticals, Inc. announced that, it has submitted a New Drug
Application (NDA) to the U.S. Food and Drug Administration (FDA) for
AMX0035 (sodium phenylbutyrate (PB) and taurursodiol (TURSO; also known
as ursodoxicoltaurine)) for the treatment of amyotrophic lateral
sclerosis (ALS).
“We
are excited to share with the ALS community the exciting milestone that
we have submitted our NDA to the FDA for review,” said Justin Klee,
Co-CEO, Director and Co-Founder of Amylyx. Joshua Cohen, Co-CEO,
Chairman and Co-Founder of Amylyx added, “Our team has worked and
continues to work around the clock as we know time is of the essence for
people living with ALS and their families. We will continue to keep the
community closely updated on our progress.”
“We
will continue to work closely with the FDA throughout the review
process to move AMX0035 toward a potential regulatory approval as
quickly and efficiently as possible,” said Tammy Sarnelli, Global Head
of Regulatory Affairs of Amylyx. “We are so inspired by the people who
participated in CENTAUR, the trial investigators, the ALS community and
our partners and team, and we will continue to work tirelessly on behalf
of you all.”
The
NDA submission to the FDA is based on data from the CENTAUR trial, a
placebo-controlled study evaluating 137 people with ALS. In this study,
participants receiving AMX0035 had statistically significant slowing of
functional decline at the end of the 6-month randomized phase as
measured by the Revised ALS Functional Rating Scale (ALSFRS-R), the most
commonly used scale in clinics worldwide to measure function in ALS. In
an interim survival analysis conducted in all randomized participants
from CENTAUR who were followed for up to three years, which included
participants who continued to receive AMX0035 in an open-label extension
phase during the follow-up period, participants who started on AMX0035
during the placebo-controlled phase of CENTAUR showed a 44% lower risk
of death compared to those who started on placebo during the
placebo-controlled phase (HR 0.56; 95% CI, 0.34-0.92). Median survival
duration through the open-label long-term follow-up phase was 25.0
months (95% CI, 19.0-33.6 months) in the group that started on AMX0035
and 18.5 months (95% CI, 13.5-23.2 months) in the group that started on
placebo, a 6.5-month difference. Overall, reported rates of adverse
events and discontinuations were substantially similar between AMX0035
and placebo groups during the 24-week randomized phase; however,
gastrointestinal events occurred with greater frequency (≥2%) in the
AMX0035 group. Detailed data from CENTAUR is published in the New England Journal of Medicine (NEJM) and Muscle and Nerve.
“This
submission brings hope to people living with ALS and their families and
caregivers,” said Merit Cudkowicz, M.D., co-principal investigator of
the CENTAUR trial and co-founder of the Northeast ALS Consortium,
Director of the Healey & AMG Center for ALS and Chair of Neurology
at Massachusetts General Hospital and the Julieanne Dorn Professor of
Neurology at Harvard Medical School. “We are honored to have led the
collaboration between the Healey & AMG Center for ALS, ALS Finding a
Cure, the ALS Association, and NEALS that made the CENTAUR trial a
reality, the efforts and results of which made this NDA submission
possible.”
“For
people living with ALS and their physicians, this is a significant
development offering hope of a potential new treatment option that has
been shown to slow ALS disease progression and extend the time families
that face this life-threatening disease have together,” said Sabrina
Paganoni, M.D., Ph.D., principal investigator of the CENTAUR trial,
investigator at the Healey & AMG Center for ALS at Massachusetts
General Hospital and Assistant Professor of PM&R at Harvard Medical
School and Spaulding Rehabilitation Hospital.
As
previously reported, Amylyx filed a New Drug Submission (NDS) for
AMX0035 for the treatment of ALS with Health Canada in June 2021. Amylyx
also intends to submit a Marketing Authorization Application (MAA) for
AMX0035 to the European Medicines Agency’s (EMA) Committee for Medicinal
Products for Human Use (CHMP) by approximately the end of 2021 and to
initiate a global Phase 3 clinical trial with sites in Europe and the
United States in the fourth quarter of 2021. The Phase 3 PHOENIX trial
of AMX0035 for the treatment of people with ALS will assess the safety
and efficacy of AMX0035 in an international population of approximately
600 participants and build upon findings from the CENTAUR trial. Amylyx
is currently exploring the possibility of an Expanded Access Program
(EAP) in the United States. If implemented, the EAP would run in
parallel with the ongoing Phase 3 PHOENIX trial and marketing
application reviews. Further information about the EAP is expected in
the fourth quarter of 2021.
More
https://en.wikipedia.org/wiki/Sodium_phenylbutyrate
https://www.kegg.jp/entry/D11836