Friday, March 22, 2019
AbbVie Announces New Drug Application Accepted for Priority Review by FDA for Upadacitinib for Treatment of Moderate to Severe Rheumatoid Arthritis

Wednesday, July 14, 2021
Drug could be promising new option against eczema
In continuation of my update on Upadacitinib
A pill called upadacitinib, already approved for treating rheumatoid arthritis, might also ease another common immunological condition—eczema.
In two phase 3 clinical trials, patients with moderate to severe eczema showed rapid and significant improvements after taking the drug, said researchers at Mount Sinai in New York City.
The clinical trials were funded by the dug's maker, AbbVie Inc., and included nearly 1,700 patients with the inflammatory skin condition.
"The results of these trials ... were so incredible that by week 16, most patients with moderate to severe atopic dermatitis [eczema] either had a 90% disease clearance, or even 100% disease clearance," study first author Dr. Emma Guttman-Yassky said in a Mount Sinai news release. She's professor and chair of the department of dermatology at Mount Sinai's Icahn School of Medicine, in New York City.
"We achieved extremely high clearance rates that are bringing us closer to the amazing clearance rates that we see in psoriasis," Guttman-Yassky noted.
According to the National Eczema Association, "people with eczema tend to have an over-reactive immune system that when triggered by a substance outside or inside the body, responds by producing inflammation. It is this inflammation that causes the red, itchy and painful skin symptoms common to most types of eczema."
Eczema affects more that 31 million American adults and between 10 to 20% of children, the study authors noted.
The two new clinical trials involved a total of almost 1,700 patients and took place between 2018 and 2020.
Besides the rapid disease clearance noted in patients, "the itch improvements already started to be significant within days from the beginning of the trials, and the maximum clinical efficacy was obtained early, at week 4, and maintained to week 16," Guttman-Yassky said.
The drug was well tolerated by patients who received the two highest doses of the drug—15 milligrams and 30 milligrams—and no significant safety risks were seen, she added.
Upadacitinib is already approved and marketed for use against rheumatoid arthritis under the brand name Rinvoq. It works by blocking what are known as multiple cytokine-signaling pathways—parts of the immune system that can malfunction and cause eczema.
According to Guttman-Yassky, other eczema therapies exist, but most come with certain drawbacks.
While injectable biologic drugs are highly successful in treating eczema patients who don't respond to or can't use topical creams, their use cannot be stopped and restarted at will, because the potential creation of anti-drug antibodies will shorten the half-life of the drugs, she explained.
However, "patients were able to start and restart [upadacitinib] at any time, allowing for flexibility, which cannot be achieved with biologics," Guttman-Yassky, said. "And, biologics, which are injectable agents that target specific lymphocytes that are 'misbehaving' or are up-regulated in atopic dermatitis, do not suppress the entire immune system as other immunosuppressants tend to do."
Dr. Michele Green is a dermatologist at Lenox Hill Hospital in New York City who wasn't involved in the new study.
She called the findings "important."
Upadacitinib is the first drug in its class "to be effectively used for patients with significant improvement of pruritus [itch] within several days of treatment and clearance of their disease within several weeks," Green noted.
"It is also significant since adolescents were included in this study and I believe an oral treatment is much more appealing to treating adolescents than current injectable biologics," she added.
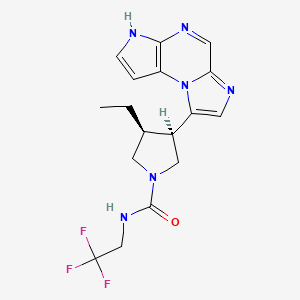
https://pubchem.ncbi.nlm.nih.gov/compound/Upadacitinib#section=2D-Structure
Thursday, January 23, 2020
FDA Approves Rinvoq (upadacitinib), an Oral JAK Inhibitor for the Treatment of Moderate to Severe Rheumatoid Arthritis

"Despite the availability of multiple treatment options with varying mechanisms of action, many patients still do not achieve clinical remission or low disease activity—the primary treatment goals for rheumatoid arthritis," said Roy M. Fleischmann, M.D., primary investigator for SELECT-COMPARE and clinical professor at the University of Texas Southwestern Medical Center at Dallas. "With this FDA approval, Rinvoq has the potential to help additional people living with RA achieve remission who have not yet reached this goal."
- In SELECT-EARLY, 52 percent of MTX-naïve patients treated with Rinvoq 15 mg achieved ACR50 vs 28 percent treated with MTX at week 121
- In SELECT-MONOTHERAPY, 68 percent of MTX-IR patients treated with Rinvoq 15 mg achieved ACR20 vs 41 percent treated with continued MTX at week 141
- In SELECT-COMPARE, 71 percent of MTX-IR patients treated with Rinvoq 15 mg plus MTX achieved ACR20 vs 36 percent treated with placebo plus MTX at week 121
- In SELECT-NEXT, 64 percent of csDMARD-IR patients treated with Rinvoq 15 mg plus csDMARDs achieved ACR20 vs 36 percent treated with placebo plus csDMARDs at week 121
- In SELECT-BEYOND, 65 percent of biologic-IR patients treated with Rinvoq 15 mg plus csDMARDs achieved ACR20 vs 28 percent treated with placebo plus csDMARDs at week 121
"The discovery and development of Rinvoq is indicative of AbbVie's long-standing commitment to advancing the science for people living with immune-mediated conditions," said Michael Severino, M.D., vice chairman and president, AbbVie. "Today's FDA approval marks an important milestone in our pursuit to deliver innovative medicines that advance care for people living with rheumatoid arthritis."
Safety
Ease of Use and Access
"Rheumatoid arthritis can have a debilitating impact on the lives of those with the chronic disease, including making it difficult to perform everyday tasks," said Cindy McDaniel, senior vice president, consumer health, Arthritis Foundation. "The Arthritis Foundation is committed to recognizing innovation that can help patients living with rheumatoid arthritis and we are proud to recognize AbbVie with our Ease of Use Commendation for the packaging design of Rinvoq."